1.0 ACPE contact hour
Faculty

Heritage Valley Health System
Beaver, PA

University of Utah School of Medicine
Salt Lake City, UT
Goal
The goal of this activity is to advance the understanding of antithrombin deficiency (ATD), and its current management.
Intended Audience
Hospital and health-system pharmacists, critical care and adult cardiothoracic anesthesiologists, hematologists, critical care, trauma, cardiovascular surgeons, and perfusionists.
Educational Objectives
After completing this activity, participants should be better able to:
- Describe ATD in terms of etiology, epidemiology, and clinical challenges.
- Review management guidelines for ATD.
- Discuss the mechanisms of action, efficacy/safety data, and clinical use of heparin and antithrombin concentrate in the management of ATD.
Physician Accreditation Statement
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Global Education Group (Global) and Applied Clinical Education (ACE). Global is accredited by the ACCME to provide continuing medical education for physicians.
Physician Credit Designation
Global designates this enduring activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Pharmacist Accreditation Statement
Global is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education with Commendation.
Pharmacist Credit Designation
Global designates this continuing education activity for 1.0 contact hour (0.1 CEU) of the ACPE (Universal Activity Number 0530-9999-24-046-H01-P).
This is a knowledge-based activity.
Global Contact Information
For information about the accreditation of this program, please contact Global at (303) 395-1782 or cme@globaleducationgroup.com.
Instructions for Obtaining Credit
To receive credit, participants must participate in the activity and complete and pass the post-test with a minimum score of 70%. Additionally, participants will need to complete the evaluation. CME certificates will be sent via email to those successfully completing the activity.
System Requirements
PC
- 1.4 GHz Intel Pentium 4 or faster processor (or equivalent)
- Windows 10, 8.1 (32-bit/64-bit); Windows 7 (32-bit/64-bit) 512 MB of RAM (1 GB recommended)
- Microsoft Internet Explorer 11 or later, Windows Edge browser, Mozilla Firefox, and Google Chrome
- For HTML Client – Google Chrome (v70.0 and above), Mozilla Firefox (v65.0 and above), and Edge (v42.0 and above)
Mac
- 1.83 GHz Intel Core Duo or faster processor 512 MB of RAM (1 GB recommended)
- MAC OS X 10.12, 10.13, and 10.14
- Mozilla Firefox, Apple Safari, Google Chrome
- For HTML Client – Google Chrome (v70.0 and above), Apple Safari (v12.0 and above), and Mozilla Firefox (v65.0 and above)
Fee Information and Refund/Cancellation Policy
There is no fee for this educational activity.
Disclosures of Relevant Financial Relationships
Global adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the Accreditation Council for Continuing Medical Education (ACCME) and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Global are required to disclose all financial relationships with any ineligible company within the past 24 months to Global. All financial relationships reported are identified as relevant and mitigated by Global in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Global to assure objectivity and that the activity is free of commercial bias. All relevant financial relationships have been mitigated.
The faculty have the following relevant financial relationships with ineligible companies:
- Stephen Bader, MD: Consultant: Medtronic, Grifols USA
- George M. Rodgers III, MD, PhD: Consulting fee (eg, advisory board): Alexion, Grifols USA, Novartis, Sanofi; contracted research (principal investigators must provide information, even if received by the institution): Swedish Orphan Biovitrum; honoraria: American Regent
- The planners and managers have the following relevant financial relationships with ineligible companies:
- The planners and managers at Global Education Group have no relevant financial relationships to disclose.
- The planners and managers at ACE have no relevant financial relationships to disclose.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents not indicated by the FDA. Global and ACE do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications on dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
false
*You must be logged in to see results data.
The function of the coagulation system is to balance bleeding and clotting. These activities depend on complex interactions among procoagulant and antithrombotic factors, including antithrombin (AT), a vitamin K-independent serine protease inhibitor (serpin). AT acts as a natural anticoagulant by inhibiting thrombin (factor IIa) and factor Xa, as well as the procoagulant factors VIIa, IXa, XIa, XIIa, kallikrein, and plasmin. Produced by the liver, AT is present in plasma, the vascular endothelium, and the extravascular space.1 In addition to its anticoagulant activity, AT also has some anti-inflammatory properties.2
Normal plasma activity levels of AT are between 80% and 120%, where 100% of AT corresponds to 1 unit of AT in 1 mL of reference plasma or a concentration of 0.125 to 0.160 mg/mL. AT deficiency (ATD) is associated with increased risk for venous thromboembolism (VTE), which includes deep vein thrombosis (DVT), pulmonary embolism (PE), and other events.3 VTE occurs in approximately 1 to 2 persons per 1000, or approximately 300,000 to 600,000 events annually.4 Even slightly decreased AT levels (80%) increase the risk for VTE (Figure 1).5 The complete absence of AT is incompatible with life.6
 |
| Figure 1. Risk for recurrent venous thromboembolism by antithrombin level.5 |
ATD can be hereditary or acquired. Hereditary ATD is the most severe inherited thrombophilia, with levels typically reduced to 40% to 60% of normal. Acquired ATD can occur in patients receiving heparin anticoagulation and in cases of trauma, pregnancy, sepsis, or severe COVID-19, making the treatment of those conditions more difficult.7 Regardless of etiology, ATD can complicate procedures such as cardiopulmonary bypass (CPB) or extracorporeal membrane oxygenation (ECMO), in which effective, high doses of heparin are essential.8 ATD is commonly underdiagnosed and sometimes only manifests when resistance to anticoagulation with heparin is noted.
In the United States, human plasma-derived AT concentrate (hpATc; Thrombate III) and recombinant antithrombin concentrate (rATc, ATryn) are approved by the FDA for the prevention of VTE in patients with hereditary ATD.9,10 Additional ATcs are approved in other countries, and many additional off-label uses have been described.7,11
This review presents the current knowledge of epidemiology, pathogenesis, diagnosis, clinical effects, and management of ATD, including in high-risk situations.
Of the normal physiologic anticoagulant effect against thrombin, 80% depends on AT, which binds directly to thrombin. Inhibition of thrombin prevents the conversion of fibrinogen to fibrin and decreases platelet and endothelial cell activation. AT also binds to factor Xa, preventing the conversion of prothrombin to thrombin, thus further inhibiting clot formation (Figure 2).7
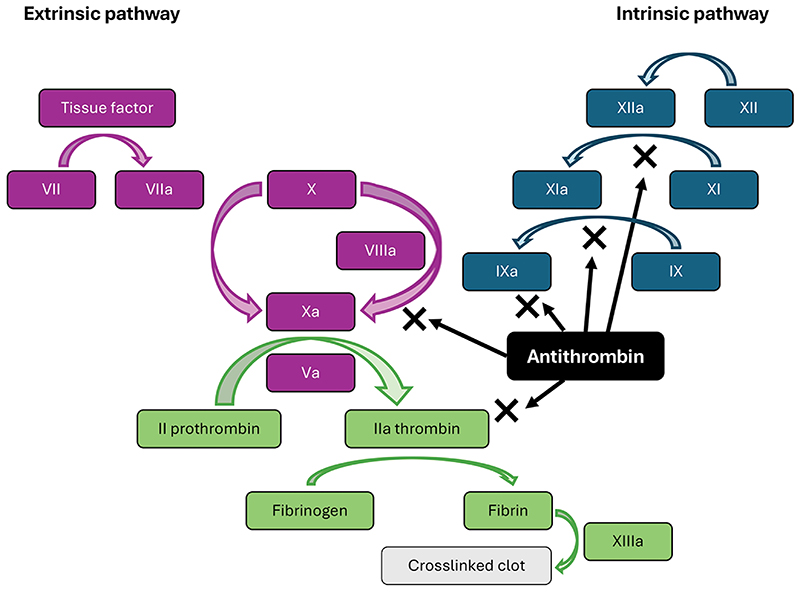 |
| Figure 2. Roles of antithrombin in extrinsic and intrinsic coagulation pathways. |
Antithrombin is a 58-kDa serine protease inhibitor encoded by the SERPINC1 gene and produced in the liver. It has a circulating half-life in plasma of approximately 3 days. The inhibitory capacity of most anticoagulant SERPINs, including AT, is enhanced by binding to negatively charged glycosaminoglycans, such as heparin.12 To function effectively as an anticoagulant, heparin and low-molecular-weight heparin (LMWH) require functional AT. Heparins are bound to AT through a common sequence-specific pentasaccharide through exposure to the AT reactive site and subsequent activation of AT. This increases AT’s inhibitory effect by more than 1000-fold through a conformational change that forms a bridge between AT and thrombin and rapidly inhibits several coagulation proteases.7,13
The anti-inflammatory capacity of AT has been known for some time. Recent investigations have found 2 mechanisms of action (MOAs). The binding of AT to specific cellular receptors reduces the proinflammatory response by inhibiting cytokine expression, and binding to endothelial cell receptors results in the release of prostaglandin I2, which restricts platelet rolling and adhesion and suppresses neutrophil interactions with the endothelium.14
Deficiency in AT can be hereditary or acquired. Hereditary ATD is associated with a very high lifetime risk for VTE. Acquired ATD manifests during heparin therapy or states of hypercoagulability, such as sepsis, trauma, the postpartum period, and premature infancy.7
Hereditary ATD
Hereditary ATD, first described in 1965, is the most severe inherited thrombophilia but is often not recognized, even in patients presenting with VTE. In the general population, hereditary ATD affects approximately 0.02% to 0.2% of people but is more common in patients with VTE (1%-5%). It is usually inherited as an autosomal-dominant disease with variable penetrance. Half of patients with ATD will develop a VTE before 50 years of age; approximately 60% of VTEs will occur spontaneously, and 40% will be due to a transient risk factor.15 Pregnant individuals with a family history of ATD are at especially high risk for developing VTE during pregnancy or after giving birth, with an overall risk of 16.6% (antepartum, 7.3%; postpartum, 11.1%).16 A 300-fold increase in thrombotic risk, especially during the neonatal period and in adolescence, has been documented in children with inherited ATD.17
Genetic background, subtype, and clinical severity of subtypes vary among patients with ATD.15 More than 300 mutations in the SERPINC1 gene, located at 1q25.1, have been recognized so far, leading to a quantitative deficiency (type I) or a qualitative defect (type II) of the protein. Three subtypes of type II deficiency can be distinguished based on the associated functional defect (Table 1).18-21 Almost all type II defects are caused by missense mutations, whereas type I ATD can result from missense or null mutations.19,20 Type I results in the reduction of both the antigen levels and functional activity, whereas type II has low activity but normal AT antigen levels.21
| Table 1. Types of Inherited ATDs18-21 | ||||
| Type of deficiency | I | IIa (Reactive site) | IIb (Heparin-binding site) | IIc (Pleiotropic effect) |
|---|---|---|---|---|
| Quantitative | Qualitative | Qualitative | Qualitative | |
| Mechanism | Heterogenous, ranging from mRNA instability to impaired protein folding and intracellular retention or degradation | AT variant has impaired reactivity with the target protease, or the inhibition process is ineffective | Mutation disturbs interaction with heparin or AT activation | Mutation affects both the reactivity and heparin affinity |
| Gene variants | Nonsense, frameshift, splicing, gross gene defects, missense, small deletions or insertions | Missense (mainly in the reactive center loop) | Missense (mainly in the heparin-binding domain) | Missense (mainly in the C-sheet) |
| Clinical phenotype | High risk for VTE | Variable risk (mild to very severe) | Lower risk for VTE | Variable risk (mild to very severe) |
| AT, antithrombin; ATD, antithrombin deficiency; mRNA, messenger RNA; VTE, venous thromboembolism. | ||||
Hereditary ATD is categorized into 2 groups (types I and II) and 3 subgroups of type II. Type I typically presents with low AT levels (quantitative deficiency), decreased to about half of normal. Type II ATD presents with normal AT levels but functionally impaired AT (qualitative deficiency). Type I ATD is more common than type II in patients or families with a history of VTEs, whereas type II is more common in the general population. Patients with type I are at high risk for VTEs, whereas, depending on the subtype, type II deficiency is associated with different degrees of clinical severity.22 Type IIa, also called type II reactive site, interferes with the ability of AT to bind with its target proteases, such as thrombin, and can result in a heightened risk for VTEs. Type IIb targets the heparin-binding site, reducing the anticoagulant activity of heparin. Interestingly, this results in a lower risk for VTE, but more arterial thromboses.23,24 Finally, patients with type IIc ATD (pleiotropic effect) also tend to have more severe VTEs, as the mutation is located in an area where it results in more than 1 functional defect.7
In a cohort study of 21 families, 13 different SERPINC1 mutations were detected, several previously unknown.21 However, in 14% of patients, the ATD was unrelated to SERPINC1 mutations. Type I deficiency was seen in 44% and type II in the remainder (6% with type IIa, 11% with type IIb, 33% with type IIc, and 6% with AT Cambridge II mutation).
Mutation analysis was also completed for 31 individuals from 27 pedigrees collected through the pediatric ATD database and biorepository maintained by the International Society on Thrombosis and Haemostasis.19 Twenty-one unique mutations of SERPINC1 were identified, and the probability of VTE-free survival at 5 years was calculated as 81.3%. Patients with missense mutations had significantly higher risk than those with null mutations (92% vs 66.7%).20 Up to 20% of cases of inherited ATD do not have a SERPINC1 gene mutation. Additional studies are needed to better characterize these defects.
Acquired ATD
The most common reason for the development of acquired ATD is therapeutic heparinization. This is due to the mechanism of AT, described above. Several other clinical scenarios, ranging from infections, trauma, liver or kidney disease to pregnancy and premature birth, can also be associated with acquired ATD. The deficiency may be due to decreased production or increased loss or consumption of AT.
Impaired Production
The synthesis of AT depends on the role of hepatocytes in producing coagulation factors and inhibitors—functions that are impaired in chronic liver diseases such as cirrhosis and hepatitis. Not only is AT production affected in these scenarios, but patients can present with altered levels of both procoagulant and anticoagulant factors, potentially resulting in bleeding or thrombosis.25,26 The degree of ATD and other coagulation factors is directly related to the severity of liver disease. In a study of patients with end-stage liver disease, the activity of protein C, protein S, and AT were measured.27 Almost 90% of patients had low levels of at least 1 coagulation factor, and 70.2% were deficient for all anticoagulant proteins studied.
Newborn babies have physiologically low levels of AT, reaching adult levels at about 3 months of age. Prematurity leads to even lower levels; however, this is generally not associated with an increased risk for spontaneous thromboses.28 Nonetheless, the number of neonatal thrombotic events has markedly increased over the last decades, and about 2% to 4% of neonates will die once diagnosed with a VTE. Almost all of these events are associated with concurrent extrinsic factors, such as central venous catheter placement.29
Increased Loss
Thromboembolism associated with nephrotic syndrome is much more common in adults than children (25% vs 3%). The pathophysiology of these events is not completely understood. Although large proteins, such as AT, may be lost through urinary leakage, a multicohort study found no correlation between AT levels and plasma albumin levels or proteinuria.30 In addition, the tendency to hypercoagulopathy did not differ between patients with normal AT levels and those with ATD (defined as <70% [<0.7 IU/mL]). In short, ATD or the development of VTEs was not consistently associated with nephrotic syndrome.
Oral Contraceptives and Pregnancy
Oral contraceptives (OCPs) and hormonal replacement therapy are known risk factors for the development of VTEs, with relative risks of 3.5 and 2.35, respectively. The risk for VTE is highest in individuals with ATD who receive estrogen-based OCPs. The MOA is not well understood but is likely related to increased thrombin production and added endothelial dysfunction.31
Among individuals with a previous VTE, the risk for VTE is increased by 5- to 6-fold during pregnancy and up to 60-fold during the first 3 months after delivery. One analysis found that AT levels during pregnancy were decreased by 20% compared with baseline, with no difference among trimesters.32 More importantly, AT levels dropped precipitously to 30% below baseline after giving birth, with a nadir at 12 hours postpartum, indicating possible consumption. Most of that risk is due to inherited thrombophilia (factor V Leiden or prothrombin 20210A mutation) and the physiologically increased clotting factors, such as fibrinogen; factors VII, VIII, IX, X, and XII; and von Willebrand factor.32 However, pregnant people with ATD are at increased risk for embryo-fetal losses and obstetric complications, as well as VTEs.34,35 Several studies have attempted to determine the risk for ATD-related VTE during pregnancy. In a meta-analysis, the absolute risk was estimated at 7.3% (95% Cl, 1.8-15.6) during the antepartum period and 11.1% (95% Cl, 3.7-21.0) during the postpartum period.16
Increased Consumption
Increased consumption of AT has been described in the settings of sepsis, COVID-19 infection, and severe trauma. Pathologic states that cause increased thrombin generation lead to accelerated consumption of AT, as it permanently binds to thrombin and is then removed from the circulation.
Sepsis frequently results in disseminated intravascular coagulopathy (DIC) and associated increased mortality.36 In one study, low AT activity (<70%) in patients with sepsis predicted an almost doubling of the 28-day mortality rate.37 Patients in whom AT levels continued to decrease after clinical interventions had the highest mortality rate. Survival was also strongly associated with AT levels in a study of 164 infants and children with sepsis.38 In this study, a receiver operating characteristic analysis was performed to define an optimal AT level for survival (Figure 3). The mortality rate below this level was 41.7% for children younger than 1 year of age and 32.2% for older children.
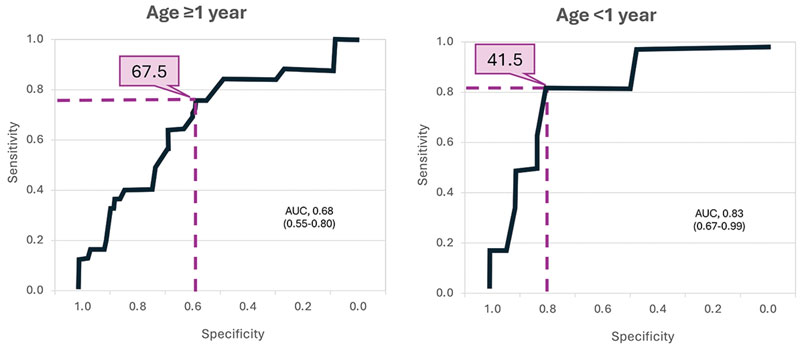 |
| Figure 3. ROC curves for survival as predicted by AT levels in pediatric patients aged ≥1 year and <1 year.38
Optimal thresholds for AT levels (%) are indicated in purple. AT, antithrombin; AUC, area under the curve; ROC, receiver operating characteristic.
Reproduced with permission. Open-access article; Creative Commons 4.0. https://creativecommons.org/licenses/by/4.0/ |
The authors of both of these studies suggested that the inclusion of AT measurements might improve the accuracy of sepsis scoring systems.37,38 Because AT activity is determined by vascular permeability, the International Society on Thrombosis and Haemostasis has proposed that AT activity levels be used as an indicator of the degree of endothelial injury, a common phenomenon in DIC associated with sepsis but less so with other infectious processes.39 DIC encountered with acute myeloid leukemia (AML) can also result in lowered AT levels.40
Insights gained from the COVID-19 pandemic raise further questions about the role of AT and its relation to patient outcomes. Severe COVID-19 illness associated with thromboembolic complications, including VTEs and strokes, resulted in high morbidity and mortality in many patients. Although coagulation abnormalities are commonly present, their effects on AT levels and activity are less clear. In one study, 3 of 36 patients with severe COVID-19 experienced a VTE, and 18 patients (50%) died. Reduced AT activity was independently associated with mortality and correlated with markers of both hypercoagulability (D-dimer) and inflammation (C-reactive protein).41 In contrast, a study of coagulation parameters in patients with acute respiratory syndrome due to COVID-19 vs other causes showed significantly higher AT levels.42 A third study found no difference in AT levels among patients with COVID-19 vs healthy controls.43
Severe trauma can trigger increased consumption of AT via a massive activation of the coagulation cascade. In addition, activated neutrophils release elastase that can degrade AT, and cardiovascular shock can impair the hepatic synthesis of AT. Low AT levels negatively affect outcomes, resulting in extended use of artificial ventilation and hospitalization in intensive care.44 In a multicenter study, trauma patients who arrived at the hospital with AT levels <80% had increased rates of shock and in-hospital mortality and needed more transfusions.45 An AT level that remained low when measured 72 hours after admission was independently associated with a 3.3-fold increased risk for developing a VTE; this is likely related to extended periods of immobilization after a major trauma.46
Other Mechanisms
Asparaginase is an enzyme commonly used as l-asparaginase or, in its pegylated form, PEG-asparaginase, in treating pediatric and adult acute lymphoblastic leukemia. Its use is known to be associated with an increased incidence of VTEs. Asparaginase decreases AT levels; however, other hemostatic factors, such as a concurrent decrease in fibrinogen, may also be involved.47-49
CPB surgery and the use of ECMO can also lead to hemostatic dysfunction, even without preexisting coagulopathy, due to stress on the body and contact between blood and the non-biologic surfaces of the circuit.50 Heparinization used during CPB or ECMO can facilitate the formation of irreversible thrombin–AT complexes, resulting in markedly decreased AT levels.
In a review of pediatric patients undergoing ECMO, the median AT activity was 65%, and ATD (levels <80%) was present in 43.3% of samples.51 The HECTIC study, which compared coagulation profiles for patients undergoing veno-venous vs veno-arterial (VA) ECMO, found that AT levels were lower in the VA group.52 This trend was also seen in another study, in which patients who spent 71% of their time on ECMO had AT levels <70%, and those who spent 11% of their time on ECMO had AT levels of <50%.53 Levels improved over time, possibly due to increased hepatic production as shock and other underlying issues resolved.
Finally, venous and arterial thromboembolic events are common in patients with cancer; however, no clear association with AT levels has been found (excluding DIC associated with AML, as described previously). In a study of 1127 patients with cancer, including 110 (9.7%) with VTEs, those with both very low and very high AT levels had poorer overall survival (u-shaped distribution).54
The most common manifestations of VTE associated with ATD are typical extremity DVT, thrombophlebitis, and PE. Less frequent complications, including splanchnic and portal vein thrombosis and arterial thrombosis, can occur as well.
Deep Vein Thrombosis
Venous thrombosis usually occurs in areas with decreased or mechanically altered blood flow, such as in proximity to valves in the deep veins of the leg. The presenting symptoms depend on the size and location of the thrombus but are mainly asymmetrical swelling, warmth, or pain in an extremity. A high index of suspicion is essential in patients with known ATD, especially those with a history of prior VTEs.55
Diagnosis is based on scoring clinical symptoms (using the Wells criteria), measurement of D-dimers, and evaluation by ultrasound (first modality), computed tomography (CT), or magnetic resonance imaging. Diagnostic accuracy of examination by ultrasound, an easily accessible modality, is estimated to be 94.2% for proximal and 63.5% for distal DVTs.56
A long-term consequence of DVT, occurring in about 50% of patients, is the development of post-thrombotic syndrome. This is usually seen within 2 years of the original DVT and is manifested by symptoms such as leg pain, swelling, and, in severe cases, venous ulcers.55,57
Pulmonary Embolism
The most common risk factor for PE is a prior history of DVT. Acute PE can range from asymptomatic to massive. Massive PE presents with hypotension or shock that results from right heart failure or cardiovascular collapse, a thrombus that occludes >50% of the pulmonary artery cross-sectional area or 2 or more lobar arteries, or if the patient is dependent on inotropic agents. On the other hand, low-risk PE is not associated with hemodynamic compromise.58
Most patients (81%) present with dyspnea, tachycardia (70%), and hypoxia (50%), but other symptoms may occur also, such as pleurisy, syncope, hypotension, and hypocapnia. CT pulmonary angiography is considered the gold standard diagnostic tool.59
Less Common Forms of VTE
Cerebral venous sinus thrombosis (CVST), often presenting with headaches or seizures, has been associated with ATD, especially during pregnancy and the postpartum period.60 Patients treated with asparaginase are also at risk for the development of CVST.49 Other uncommon forms of VTE include splanchnic thrombosis, retinal thrombosis, renal or hepatic vein thrombosis, and thrombotic events in the upper extremities. Although there are case reports associating them with ATD, other predisposing factors are often present in many instances, such as a more general coagulopathy or the use of indwelling vascular catheters.61
Arterial Thromboses
A systematic review of almost 12,000 patients with stroke revealed an increased risk for inherited thrombophilia, although the pooled odds ratio (1.73; 95% CI, 0.70-4.28) did not reach statistical significance for ATD.23 However, there might have been some bias in testing and publication in this review. Other authors have documented a high detectability of SERPINC1 mutations in patients with a stroke and documented ATD.24
In addition to personal and family history of VTE episodes, laboratory tests are usually necessary to confirm the diagnosis of ATD. These can be done by using several different assays.3,7 The 2 main types measure the activity (functional assays) or quantity of the protein (antigen immunoassay). Adult reference values usually vary between 80 and 120 IU/dL, but each laboratory should also establish its own reference range. The Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis recommends employing an activity assay for initial testing followed by an antigen test and adding a calculation of the activity-to-antigen ratio when the activity level is low.3 Figure 4 depicts the recommended approach to diagnosing ATD in adults.3,11 Notably, normal reference intervals for AT levels are higher in children than adults.7
 |
| Figure 4. Recommended approach to diagnosing AT deficiency in adults.3,11
AT, antithrombin; HBS, heparin-binding site; PE, pleotropic embolism; RS, reactive site; VTE, venous thromboembolism. |
Antithrombin activity can be measured using commercial kits through factor IIa (thrombin)- or factor Xa-based chromogenic methods. Excess thrombin or factor Xa and heparin are added to the patient’s plasma for endogenous AT to inhibit thrombin. A chromogenic peptide substrate for uninhibited thrombin is added, thrombin cleaves the substrate, and the release of the chromogen results in a measurable change in absorbance detected by a spectrophotometer. Some modifications of the assays may be needed to detect specific variants. Several methods are used to measure antigen levels, including enzyme-linked immunosorbent assay, radial immunodiffusion, and immunoelectrophoresis.3
Testing for all hereditary thrombophilias (using a panel of tests) is recommended for individuals with a family history of VTE and known AT, protein C, or protein S deficiency (high-risk thrombophilia), as per 2023 guidelines from the American Society of Hematology (ASH). This recommendation is based on the estimate that the relative risk for a first VTE in a person with a family history of AT deficiency is 12.17 (95% CI, 5.45-27.17).62
Genetic testing can be an efficient method for screening patients with relevant family history. However, screening the general population for AT deficiency is not recommended, mainly because of the variable penetrance and the fact that so many different mutations of the SERPINC1 gene have been recognized.20,21 Most type I mutations are rare, private mutations usually found in a single family, whereas a founder effect renders some type II mutations more common in certain populations.3
However, there are pros and cons related to testing for ATD (or any thrombophilia), as mentioned in the ASH guidelines:
Although testing patients with VTE or relatives of patients with VTE and thrombophilia has a moderate to high chance of finding a positive test result, suggesting that the incremental value of knowing about the presence or absence of thrombophilia may be low. Thrombophilia testing can lead to overdiagnosis, defined as the labeling of a person with a disease or abnormal condition that would not have caused the person clinical harm if left undiscovered, [but] they may experience physical, psychological, or financial harm if the condition is discovered.62
Monitoring anticoagulation during CPB or ECMO is associated with unique challenges, as AT levels are not the only factor contributing to the risk for bleeding or thrombosis. Table 2 lists the currently used methods.3,50
| Table 2. Assays Used to Monitor Anticoagulation During ECMO or CPB3,5 | ||
| Test | Technique | Comment |
|---|---|---|
| Activated clotting time | Measurement of time to formation of initial fibrin clot after adding whole blood to a tube containing a surface activator, resulting in the stimulation of the contact activation pathway | Poor correlation with heparin and anti-Xa levels (especially for lower ranges as used for ECMO) |
| Activated partial thromboplastin time | >300 laboratory methods used with different results | |
| Anti-factor Xa | Direct measurement of inhibition of factor Xa by heparin | |
| Thromboelastogram or rotational elastometry | Viscoelastic tests that measure time to initial fibrin formation, crosslinking of fibrin, clot firmness, platelet function, and fibrinolysis | No large comparative trials available |
| Antithrombin | Difficult to interpret, as part of a greater coagulation disturbance during ECMO or CPB surgery | |
| CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; ELISA, enzyme-linked immunosorbent assay. | ||
A personalized approach is essential in the management of ATD. Three scenarios are commonly encountered, each with its own challenges: treating an acute VTE, providing short-term thromboprophylaxis for patients in high-risk clinical settings, and long-term anticoagulant thromboprophylaxis for symptomatic patients.7 Special challenges arise when managing pregnant patients with either inherited or acquired ATD or when performing CPB or using ECMO.
Treating an Acute VTE
For the treatment of an acute VTE, the 2024 compendium of guidelines from the American College of Chest Physicians (ACCP) state tha t LMWH or fondaparinux, a factor Xa inhibitor related to heparin, are preferred over intravenously or subcutaneously administered unfractionated heparin (UFH). In patients with a high suspicion of VTE, parenteral anticoagulation should be initiated promptly while awaiting the results of diagnostic tests, even if they are currently on a vitamin K antagonist (VKA).63,64 In patients with an acute VTE, the direct-acting oral anticoagulants (DOACs) apixaban, dabigatran, edoxaban, and rivaroxaban are recommended over warfarin.64 In the presence of known ATD, additional treatment with ATcs or fresh frozen plasma (FFP) might be indicated, as the efficiency of heparin is dependent on AT.65
Short-Term Thromboprophylaxis
Short-term thromboprophylaxis is recommended for asymptomatic patients with ATD in high-risk situations, such as surgery and pregnancy. The risk for clotting, especially DVTs, is increased after surgery due to the stimulation of prothrombotic factors, increased blood stasis, and prolonged immobility.66 It has been suggested that replacement therapy be used to bring AT levels into the normal range (80%-120%) and LMWH be used at usual prophylactic doses. In patients at very high risk for thrombosis, such as those undergoing cancer surgery or cesarean delivery, AT replacement is recommended for approximately 5 days to maintain AT levels >70%.15 Pregnancy in people with a history of a prior VTE is associated with especially high risk.34 AT concentrates (ATcs) have been approved for the prevention of perioperative and peripartum thrombosis in patients with hereditary AT.
Long-Term Anticoagulant Thromboprophylaxis
Patients with ATD have a high risk for developing recurrent VTEs (13%-17% per year), and long-term anticoagulation should be strongly considered for these individuals. The VKA warfarin or DOACs have been used successfully; the recent ACCP guidelines recommend the use of a DOAC, such as apixaban, dabigatran, edoxaban, or rivaroxaban rather than anticoagulation with a VKA.64 In a recent prospective cohort study comparing the use of DOACs with heparin or VKA in 29 patients with inherited thrombophilia and ATD, the efficacy of the 2 treatment approaches was similar. Bleeding events were more frequent in the DOAC group (10.2% vs 4.97%), but none were major.67
Anticoagulation During Pregnancy and Delivery
Pregnancy and the postpartum period, especially the first few weeks postpartum, are the highest risk periods for the development of a VTE. VTEs can even occur during the first trimester, necessitating early intervention in many cases.34,35 For at-risk individuals, thromboprophylaxis with LMWH is recommended during pregnancy, as heparin does not cross the placental barrier or pass into breast milk. If anticoagulation represents too great a risk for bleeding (surgery, childbirth) or in the case of an actual VTE despite anticoagulation, replacement therapy with ATc is recommended. Unfortunately, guidelines around dosing and timing of anticoagulation are not consistent, and it is recommended that persons with known ATD be managed in collaboration with a specialized center during pregnancy.34
Anticoagulation During ECMO or CPB
Anticoagulation with UFH is the standard of care for patients undergoing CPB or ECMO. However, because the activity of heparin depends on adequate AT levels, the patient might develop an altered heparin response or heparin resistance and require high doses to achieve adequate anticoagulation, as measured by a therapeutic activated partial thromboplastin time or anti-Xa level.8 Preoperative heparin infusions are commonly used in patients requiring heart surgery or ECMO, leading to a high prevalence of acquired ATD. Only a small group of patients will develop clinically significant heparin resistance that requires active intervention.
Replacement therapy with ATc or transfusion of FFP are alternatives. Of note, FFP transfusion is associated with several risks, such as potential circulatory overload (especially in neonates or patients with renal compromise), allergic reaction, transfusion-related acute lung injury (TRALI), transmission of infections, hemolytic transfusion reaction, and alloimmunization.68 The minimal AT activity required for an adequate heparin effect is not known. Furthermore, infants and neonates have physiologically lower AT levels and age-appropriate thresholds of minimally active AT levels are lacking.50 Despite the absence of randomized studies, many clinicians prefer using a direct thrombin inhibitor, such as argatroban or bivalirudin, rather than heparin.69
The FDA has approved 2 ATcs for the prevention of VTEs associated with inherited ATD. One is derived from human plasma, and the other is produced through recombinant techniques. The plasma-derived ATc is also approved for the treatment of VTEs in the same population.
Human Plasma-Derived ATc
The only brand of hpATc approved in the United States for the treatment of thromboembolism and prevention of perioperative and peripartum thromboembolism in patients with hereditary ATD is Thrombate III.9 This product is a lyophilized preparation of AT pooled from human plasma that contains no preservatives. To ensure safety, it is fractionated and heated to destroy infectious agents without diminishing biological activity, and no infections, including with the prions causing Creutzfeldt-Jacob disease, have been reported.9
Treatment with hpATc should be administered within 3 hours of reconstitution and given intravenously over a period of 10 to 20 minutes.9 Dosing is calculated using the following formula:
| Units required (IU) | = | 120% – baseline % × body weight (kg) 1.4% |
Administration of 1 IU/kg of body weight increases plasma AT activity by 1.5%. After an initial loading dose to reach an AT level of 120%, AT levels should be measured every 12 hours, and a maintenance dose (approximately 60% of the initial dose) should be given every 24 hours as needed to maintain AT levels between 80% and 120%. The product has a prolonged half-life of 2.5 to 3.8 days.9
A population-based study evaluated the pharmacokinetics (PK) of hpATc in 184 children who were not receiving mechanical circulatory support.70 The study showed that concurrent dosing with unfractionated heparin and pre-dose AT activity levels are significant PK covariates that may affect AT clearance and volume of distribution, respectively. Another PK study involved retrospective review of hpATc in 83 pediatric patients (half of them neonates) on ECMO.71 The amount of bioavailable AT was reduced to 50% during ECMO vs non-ECMO conditions, resulting in a doubling of clearance, central and peripheral volume, and intercompartmental clearance. The authors suggested that the goal of AT replacement should be 50% to 80% for neonates and 80% to 120% for infants and children older than 30 days.
In an efficacy study, none of the 10 patients with inherited ATD treated prophylactically with hpATc developed a VTE; this included 4 pregnant women.72 Another 5 individuals who were treated for an acute VTE recovered without further thrombotic extension or recurrence. Similar results were found in 9 patients with acquired ATD. Administration of hpATc was well tolerated, with the most common side effects being dizziness, chest discomfort, nausea, dysgeusia, and pain (cramps).
Similarly, no side effects were reported in another study of 18 patients with hereditary ATD.73 In the 8 asymptomatic patients, the AT level increase per kilogram body weight ranged from 1.56% to 2.74% with a half-life of 43.3 to 77 hours. In clinically ill patients, the efficacy was somewhat lower, with an increase of 0.64% to 1.90% per unit hpATc infused/kg. No VTEs were observed in either group.
Experimental ATc
Another human plasma-derived AT product (Atenativ) is approved in countries outside the United States and is currently in phase 3 studies in US patients planning to undergo surgery or delivery (NCT04918173) and individuals with heparin resistance scheduled for cardiac surgery (NCT06096116).
Recombinant AT
The rAT product available in the United States (ATryn) is produced from transgenic goats who express AT in their milk and is FDA-approved for the prevention—but not treatment—of perioperative and peripartum thromboembolic events in hereditary ATD.10 The PK profile of rAT is different from that of hpATc. A population PK study in patients with hereditary AT deficiency determined that different dosing should be used in surgical patients vs pregnant patients receiving this product70,74 (Table 3).
| Table 3. Dosing for Recombinant Antithrombin Concentrate10,7 | ||
| Loading dose (IU) | Maintenance dose (IU/h) | |
|---|---|---|
| Surgical patients | (100 – baseline AT activity) × body weight (kg) 2.3 | (100 – baseline AT activity) × body weight (kg) 10.2 |
| Pregnant patients | (100 – baseline AT activity) × body weight (kg) 1.3 | (100 – baseline AT activity) × body weight (kg) 5.4 |
| AT, antithrombin. | ||
A study of 5 patients with inherited AT undergoing surgery did not show any VTEs, and no antibodies were found in the 4 patients tested.75 Another retrospective review included 77 neonatal and pediatric patients treated with ECMO and receiving rATc to maintain an AT activity level of >80%. The duration on ECMO was the main predictor for thrombotic events, and there was no correlation with AT activity levels as measured by anti-Xa activity.76 In a prospective, randomized, placebo-controlled study, no benefit or toxicity was noted when rAT was administered to women with signs of pre-eclampsia.77
In a phase 3, double-blind, placebo-controlled study of heparin-resistant patients undergoing cardiac surgery with CPB, FFP was administered if the activated clotting time remained <480 seconds despite the administration of rAT or placebo. This was necessary in 19% of patients in the rAT group and 81% of patients in the placebo group. There was no difference in adverse events between the 2 groups.78
The rAT product is contraindicated in patients with a goat or goat milk allergy, and patients need to be monitored closely for hypersensitivity reactions. Because of its shorter half-life compared with hpAT (11.6-17.7 hours), it should be administered as an initial bolus followed by continuous infusion. AT levels should be monitored 2 hours after the initial dose or 2 hours after each adjustment.10,15 This product also has a greater affinity to heparin compared with hpATc,79 and thus may exhibit hemorrhagic effects if the dosage is not optimized.80
Another product uses a different technology to produce recombinant human AT-gamma, a fucose-free recombinant protein, from Chinese hamster ovary cells. Licensed in Japan, this formulation is more similar to hpATc than rAT, including a longer half-life.81
FFP is the other commonly used replacement product; however, ATc delivers 50 times more AT than the same amount of FFP, and because FFP has a lower concentration of AT per volume, it requires administration of a higher infusion volume, increasing risk for volume overload.64,82 FFP-associated hemodilution may lead to transfusion of additional blood products, specifically packed red cells. Excess red cell transfusion has been associated with increased mortality in cardiac surgery. Moreover, FFP may not be effective at achieving adequate heparinization and restoring activated clotting times (ACTs).61 In contrast, ATcs are associated with lower infusion volume and a reduced risk for TRALI and infusion-related reactions.61 An ATc delivers a more predictable amount of AT with a more predictable half-life.82-85
In a review of the medical records of 502 patients who underwent CPB and received either ATc (n=247 patients) or FFP (n=255), the use of ATc was associated with a greater benefit.86 The ATc cohort had a 71% reduction in mortality (odds ratio, 0.29) compared with the FFP group, a 22% shorter ICU length of stay, and 10% more hospital-free days in the 30 days after discharge.
ATC also has practical advantages over FFP. Despite visual inspection, FFP bags may contain small, imperceptible holes or tears, leading to a bag breakage rate of 12.6% at one institution.87 Whereas ATcs can be stored at room temperature at the point of care, FFP must be stored frozen (at –18°C, 0°F) and transported from the blood bank to the operating room. The thawing process can take at least 20 minutes, followed by another 8 minutes to achieve the therapeutic ACT.61 Across hundreds of cases, the aggregate price of these delays can become significant, considering an average operating room cost of $46 per minute.61,88 In fact, from a provider perspective, the total cost of FFP administration was nearly 10 times higher than the initial acquisition cost in a recent report.89
As discussed, ATcs are often used off-label in patients on ECMO or undergoing CPB for heparin resistance, which is observed as the need for higher UFH doses. Heparin polymers bind to AT and accelerate the interaction with thrombin or factor Xa, thus inhibiting the prothrombotic effect. A similar effect is seen in patients with endogenously low AT levels, whether acquired or inherited. Infused ATc binds and inactivates many clotting factors in the coagulation cascade, leading to their rapid clearance and allowing heparin to recirculate.8 However, debate about the role of AT replacement in ECMO and CPB is ongoing. In a review of practices in 50 countries, AT activity was measured in fewer than half (48.7%) of the patients undergoing veno-venous ECMO.90 AT supplementation was routinely prescribed in 38.1% of centers, the majority in high-income countries. The Pediatric ECMO Anticoagulation Collaborative reviewed the available data and did not find sufficient evidence to provide recommendations on monitoring and replacement therapy with ATc in children on ECMO.91
The hpATc has also been studied as a preoperative supplementation in cardiac surgery with CPB.92 In this study, 425 patients were randomized to receive either placebo or a single dose of hpATc before surgery, to achieve an absolute increase of 20% above pretreatment AT activity. AT activity was significantly higher in the AT group (108%) vs the placebo group (76%), an effect that lasted until postoperative day 2. However, there was no difference between the 2 groups on the major morbidity composite, which included postoperative mortality, stroke, acute kidney injury, need for surgical reexploration, arterial or venous thromboembolic events, prolonged mechanical ventilation, and infection.
Multiple other potential indications have been explored, including veno-occlusive disease, during continuous renal replacement therapy, in children with chylothorax, and in the treatment of DIC associated with sepsis. However, the results are mixed, and more studies are needed.7 For example, adding hpATc to thrombomodulin in patients with DIC and sepsis showed a benefit in patients who had both low platelets and low AT levels.93
There is a paucity of studies comparing hpATc with rATc. A randomized PK study in healthy volunteers showed bioequivalence between rATc-gamma, the formulation approved in Japan, at 72 IU/kg vs hpATc at a dose of 60 IU/kg.81 A study in patients with sepsis-related DIC showed similar activity and safety between the 2 products.94 Another study in patients with sepsis and DIC and multiple organ failure appeared to show a benefit of rATc over hpATc; however, this study was performed in Japan, where hpATc is not given based on body weight but as a standard dose of 1,500 IU/day for 3 days, and rATc dosing is based on body weight.95 The authors hypothesized that the fixed dose of hpATc did not provide the same capability to reach therapeutic plasma levels, and that patients benefit from per-kilogram dosing for both preparations.
Case 1: A 19-year-old woman was evaluated for possible inherited ATD due to a positive family history of VTE. Her mother and aunt had a history of thrombosis, and an investigation into hereditary thrombophilia revealed low blood AT activity in both. Furthermore, her grandmother died after a severe thrombotic event. The patient was subsequently diagnosed as positive for a genetic mutation that confirmed hereditary AT deficiency and was found to have 60% baseline AT activity despite never having experienced thrombosis.
At a subsequent follow-up visit, she was found to be pregnant and, given that her mother and grandmother both experienced complications during pregnancy, was started on AT replacement therapy with hpATc along with a LMWH, enoxaparin. After daily doses, the regimen was adjusted to every other day once AT activity was stabilized in the range of 80% to 120% and then continued for several weeks postpartum to enhance AT activity further.
Case 2: An 18-year-old man was referred for an abdominoperineal resection for long-standing intractable Crohn’s disease. This is considered major abdominal surgery. At assessment, a degree of ATD was noted and found to be at 70% baseline activity. It is likely that acquired ATD may have been triggered by lifelong chronic illness despite this patient never experiencing a thrombotic episode. No family history of ATD or thromboembolic disease was identified by caregivers.
Given the dual stressors of severe gastrointestinal disease and the anticipated surgery, it was determined that presurgical AT replacement therapy should be initiated as thromboprophylaxis. This consisted of hpATc along with enoxaparin as LMWH. The supplementation was continued for 2 weeks postoperatively, along with careful monitoring for several weeks thereafter to ensure improvement of AT activity, which, over time, eventually allowed for an uneventful recovery.
Antithrombin plays an important role in the coagulation cascade, and its activity is greatly enhanced in the presence of heparin. Although often undiagnosed, patients can have several forms of inherited or acquired ATD, resulting in different degrees of risk for developing VTEs or even arterial thromboses. Diagnosis relies on obtaining a thorough family and personal history, quantitative and qualitative tests, and, in some cases, genetic testing.
Replacement therapy with either a human plasma-derived product or one created with recombinant technology is approved for the prevention (both products) or treatment (only hpATc) of VTEs in patients with hereditary ATD. Many other potential indications are currently being explored, but none have shown strong evidence for its use. There is a need for more rigorous investigations and broader education about ATD, as the occurrence of VTEs is a growing problem.
- Carlson TH, Simon TL, Atencio AC. In vivo behavior of human radioiodinated antithrombin III: distribution among three physiologic pools. Blood. 1985;66(1):13-19.
- Rezaie AR, Giri H. Antithrombin: an anticoagulant, anti-inflammatory and antibacterial serpin. J Thromb Haemost. 2020;18(3):528-533.
- Van Cott EM, Orlando C, Moore GW, et al. Recommendations for clinical laboratory testing for antithrombin deficiency; communication from the SSC of the ISTH. J Thromb Haemost. 2020;18(1):17-22.
- Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693-4738.
- Sokol J, Timp JF, le Cessie S, et al. Mild antithrombin deficiency and risk of recurrent venous thromboembolism: results from the MEGA follow-up study. J Thromb Haemost. 2018;16(4):680-688.
- Ishiguro K, Kojima T, Kadomatsu K, et al. Complete antithrombin deficiency in mice results in embryonic lethality. J Clin Invest. 2000;106(7):873-878.
- Rodgers GM, Mahajerin A. Antithrombin therapy: current state and future outlook. Clin Appl Thromb Hemost. 2023;29:10760296231205279.
- Levy JH, Connors JM. Heparin resistance - clinical perspectives and management strategies. N Engl J Med. 2021;385(9):826-832.
- THROMBATE III (antithrombin III [human]) prescribing information. Research Triangle Park, NC: Grifols Therapeutics LLC; 2021. Accessed October 22, 2024. https://www.drugs.com/pro/thrombate-iii.html
- ATryn (antithrombin [recombinant]) prescribing information. Deerfield, IL: Ovation Pharmaceuticals, Inc. 2009. Accessed October 22, 2024. https://www.fda.gov/media/75529/download
- Rodgers GM. Role of antithrombin concentrate in treatment of hereditary antithrombin deficiency. An update. Thromb Haemost. 2009;101(5):806-812.
- Grover SP, Mackman N. Anticoagulant SERPINs: endogenous regulators of hemostasis and thrombosis. Front Cardiovasc Med. 2022;9:878199.
- Olson ST, Richard B, Izaguirre G, Schedin-Weiss S, Gettins PG. Molecular mechanisms of antithrombin-heparin regulation of blood clotting proteinases. A paradigm for understanding proteinase regulation by serpin family protein proteinase inhibitors. Biochimie. 2010;92(11):1587-1596.
- Schlömmer C, Brandtner A, Bachler M. Antithrombin and its role in host defense and inflammation. Int J Mol Sci. 2021;22(8):4283.
- Pabinger I, Thaler J. How I treat patients with hereditary antithrombin deficiency. Blood. 2019;134(26):2346-2353.
- Croles FN, Nasserinejad K, Duvekot JJ, Kruip MJ, Meijer K, Leebeek FW. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: systematic review and Bayesian meta-analysis. BMJ. 2017;359:j4452.
- de la Morena-Barrio B, Orlando C, de la Morena-Barrio ME, Vicente V, Jochmans K, Corral J. Incidence and features of thrombosis in children with inherited antithrombin deficiency. Haematologica. 2019;104(12):2512-2518.
- Bravo-Pérez C, de la Morena-Barrio ME, Vicente V, Corral J. Antithrombin deficiency as a still underdiagnosed thrombophilia: a primer for internists. Pol Arch Intern Med. 2020;130(10):868-877.
- Corral J, de la Morena-Barrio ME, Vicente V. The genetics of antithrombin. Thromb Res. 2018;169:23-29.
- Kumar R, Bakeer N, Dawson J, et al. Impact of SERPINC1 mutation on thrombotic phenotype in children with congenital antithrombin deficiency-first analysis of the International Society on Thrombosis and Haemostasis pediatric antithrombin deficiency database and biorepository. J Thromb Haemost. 2023;21(5):1248-1257.
- Mulder R, Croles FN, Mulder AB, Huntington JA, Meijer K, Lukens MV. SERPINC1 gene mutations in antithrombin deficiency. Br J Haematol. 2017;178(2):279-285.
- Tait RC, Walker ID, Perry DJ, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994;87(1):106-112.
- Chiasakul T, De Jesus E, Tong J, et al. Inherited thrombophilia and the risk of arterial ischemic stroke: a systematic review and meta-analysis. J Am Heart Assoc. 2019;8(19):e012877.
- Kim S, Lee WJ, Moon J, Jung KH. Utility of the SERPINC1 gene test in ischemic stroke patients with antithrombin deficiency. Front Neurol. 2022;13:841934.
- Lisman T, Leebeek FW. Hemostatic alterations in liver disease: a review on pathophysiology, clinical consequences, and treatment. Dig Surg. 2007;24(4):250-258.
- Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost. 2011;9(9):1713-1723.
- Singhal A, Karachristos A, Bromberg M, Daly E, Maloo M, Jain AK. Hypercoagulability in end-stage liver disease: prevalence and its correlation with severity of liver disease and portal vein thrombosis. Clin Appl Thromb Hemost. 2012;18(6):594-598.
- Neary E, McCallion N, Kevane B, et al. Coagulation indices in very preterm infants from cord blood and postnatal samples. J Thromb Haemost. 2015;13(11):2021-2030
- Khizroeva J, Makatsariya A, Vorobev A, et al. The hemostatic system in newborns and the risk of neonatal thrombosis. Int J Mol Sci. 2023;24(18):13864.
- Abdelghani E, Waller AP, Wolfgang KJ, et al. Exploring the role of antithrombin in nephrotic syndrome-associated hypercoagulopathy: a multi-cohort study and meta-analysis. Clin J Am Soc Nephrol. 2023;18(2):234-244.
- Pastori D, Cormaci VM, Marucci S, et al. A comprehensive review of risk factors for venous thromboembolism: from epidemiology to pathophysiology. Int J Mol Sci. 2023;24(4):3169.
- James AH, Rhee E, Thames B, Philipp CS. Characterization of antithrombin levels in pregnancy. Thromb Res. 2014;134(3):648-651.
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6(4):632-637.
- Hart C, Rott H, Heimerl S, Linnemann B. Management of antithrombin deficiency in pregnancy. Hamostaseologie. 2022;42(5):320-329.
- Pabinger I, Vormittag R. Thrombophilia and pregnancy outcomes. J Thromb Haemost. 2005;3(8):1603-1610.
- Gando S, Shiraishi A, Yamakawa K, et al. Role of disseminated intravascular coagulation in severe sepsis. Thromb Res. 2019;178:182-188.
- Wei Q, Wang M, Peng X, Yang J, Niu T. Comparison of three different disseminated intravascular coagulation (DIC) criteria and diagnostic and prognostic value of antithrombin investigation in patients with confirmed sepsis-induced coagulopathy (SIC). Clin Appl Thromb Hemost. 2024;30:10760296241271334.
- Niederwanger C, Hell T, Hofer S, et al. Antithrombin deficiency is associated with mortality and impaired organ function in septic pediatric patients: a retrospective study. PeerJ. 2018;6:e5538.
- Iba T, Levy JH, Thachil J, Susen S, Levi M, Scarlatescu E. Communication from the Scientific Standardization Committees of the International Society on Thrombosis and Haemostasis on vascular endothelium-related biomarkers in disseminated intravascular coagulation. J Thromb Haemost. 2023;21(3):691-699.
- Yoshinobu S, Honda G, Kawano N, et al. Clinical features of disseminated intravascular coagulation according to the French-American-British classification in patients with acute leukemia and thrombomodulin alfa treatment-a cohort study using a postmarketing surveillance database. Clin Appl Thromb Hemost. 2021;27:10760296211054094.
- Chen-Goodspeed A, Dronavalli G, Zhang X, et al. Antithrombin activity is associated with persistent thromboinflammation and mortality in patients with severe COVID-19 illness. Acta Haematol. 2023;146(2):117-124.
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-1098.
- Ueland T, Michelsen AE, Tveita AA, et al. Coagulopathy and adverse outcomes in hospitalized patients with COVID-19: results from the NOR-Solidarity trial. Res Pract Thromb Haemost. 2023;8(1):102289.
- Farrell DH, McConnell KM, Zilberman-Rudenko J, et al. Antithrombin III levels and outcomes among patients with trauma. JAMA Netw Open. 2024;7(8):e2427786.
- Wada T, Shiraishi A, Gando S, et al. Association of antithrombin with development of trauma-induced disseminated intravascular coagulation and outcomes. Front Immunol. 2022;13:1026163.
- Rahbar E, Cotton BA, Wade CE, Cardenas JC. Acquired antithrombin deficiency is a risk factor for venous thromboembolism after major trauma. Thromb Res. 2021;204:9-12.
- Rank CU, Toft N, Tuckuviene R, et al. Thromboembolism in acute lymphoblastic leukemia: results of NOPHO ALL2008 protocol treatment in patients aged 1 to 45 years. Blood. 2018;131(22):2475-2484.
- Ruiz-Llobet A, Gassiot S, Sarrate E, et al. Venous thromboembolism in pediatric patients with acute lymphoblastic leukemia under chemotherapy treatment. Risk factors and usefulness of thromboprophylaxis. Results of LAL-SEHOP-PETHEMA-2013. J Thromb Haemost. 2022;20(6):1390-1399.
- Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia. 2013;27(3):553-559.
- Chlebowski MM, Baltagi S, Carlson M, Levy JH, Spinella PC. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. 2020;24(1):19.
- Procaccini DE, Roem J, Ng DK, et al. Evaluation of acquired antithrombin deficiency in paediatric patients supported on extracorporeal membrane oxygenation. Br J Clin Pharmacol. 2023;89(8):2396-2406.
- Cartwright B, Bruce HM, Kershaw G, et al. Hemostasis, coagulation and thrombin in venoarterial and venovenous extracorporeal membrane oxygenation: the HECTIC study. Sci Rep. 2021;11(1):7975.
- Mansour A, Berahou M, Odot J, et al. Antithrombin levels and heparin responsiveness during venoarterial extracorporeal membrane oxygenation: a prospective single-center cohort study. Anesthesiology. 2024;140(6):1153-1164.
- Englisch C, Königsbrügge O, Nopp S, et al. Antithrombin activity and association with risk of thrombosis and mortality in patients with cancer. Int J Mol Sci. 2022;23(24):15770.
- Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
- Goodacre S, Sampson F, Thomas S, van Beek E, Sutton A. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005;5:6.
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135(5):317-325.
- Marginean A, Arora P, Walsh K, et al. Utilization of a novel scoring system in predicting 30-day mortality in acute pulmonary embolism, the CLOT-5 pilot study. Clin Appl Thromb Hemost. 2024;30:10760296241278353.
- Martinez Licha CR, McCurdy CM, Maldonado SM, Lee LS. Current management of acute pulmonary embolism. Ann Thorac Cardiovasc Surg. 2020;26(2):65-71.
- Uluduz D, Sahin S, Duman T, et al. Cerebral venous sinus thrombosis in women: subgroup analysis of the VENOST study. Stroke Res Treat. 2020;2020:8610903.
- Abbattista M, Capecchi M, Martinelli I. Treatment of unusual thrombotic manifestations. Blood. 2020;135(5):326-334.
- Middeldorp S, Nieuwlaat R, Baumann Kreuziger L, et al. American Society of Hematology 2023 guidelines for management of venous thromboembolism: thrombophilia testing. Blood Adv. 2023;7(22):7101-7138.
- Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225.
- Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: compendium and review of CHEST guidelines 2012-2021. Chest. 2024;166(2):388-404.
- Nair PM, Rendo MJ, Reddoch-Cardenas KM, Burris JK, Meledeo MA, Cap AP. Recent advances in use of fresh frozen plasma, cryoprecipitate, immunoglobulins, and clotting factors for transfusion support in patients with hematologic disease. Semin Hematol. 2020;57(2):73-82.
- Evtugina NG, Peshkova AD, Pichugin AA, Weisel JW, Litvinov RI. Impaired contraction of blood clots precedes and predicts postoperative venous thromboembolism. Sci Rep. 2020;10(1):18261.
- Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9(23):e018917.
- Beattie GW, Jeffrey RR. Is there evidence that fresh frozen plasma is superior to antithrombin administration to treat heparin resistance in cardiac surgery? Interact Cardiovasc Thorac Surg. 2014;18(1):117-120.
- M’Pembele R, Roth S, Metzger A, et al. Evaluation of clinical outcomes in patients treated with heparin or direct thrombin inhibitors during extracorporeal membrane oxygenation: a systematic review and meta-analysis. Thromb J. 2022;20(1):42.
- Moffett BS, Diaz R, Galati M, Mahoney D, Teruya J, Yee DL. Population pharmacokinetics of human antithrombin concentrate in paediatric patients. Br J Clin Pharmacol. 2017;83(11):2450-2457.
- Jung D, Procaccini D, Roem J, et al. Pharmacokinetics of human plasma-derived antithrombin in pediatric patients supported on extracorporeal membrane oxygenation. J Clin Pharmacol. 2024;64(11):1382-1390.
- Schwartz RS, Bauer KA, Rosenberg RD, Kavanaugh EJ, Davies DC, Bogdanoff DA. Clinical experience with antithrombin III concentrate in treatment of congenital and acquired deficiency of antithrombin. The Antithrombin III Study Group. Am J Med. 1989;87(3B):53S-60S.
- Menache D, O’Malley JP, Schorr JB, et al. Evaluation of the safety, recovery, half-life, and clinical efficacy of antithrombin III (human) in patients with hereditary antithrombin III deficiency. Cooperative Study Group. Blood. 1990;75(1):33-39.
- DeJongh J, Frieling J, Lowry S, Drenth HJ. Pharmacokinetics of recombinant human antithrombin in delivery and surgery patients with hereditary antithrombin deficiency. Clin Appl Thromb Hemost. 2014;20(4):355-364.
- Konkle BA, Bauer KA, Weinstein R, Greist A, Holmes HE, Bonfiglio J. Use of recombinant human antithrombin in patients with congenital antithrombin deficiency undergoing surgical procedures. Transfusion. 2003;43(3):390-394.
- Todd Tzanetos DR, Myers J, Wells T, Stewart D, Fanning JJ, Sullivan JE. The use of recombinant antithrombin III in pediatric and neonatal ECMO patients. ASAIO J. 2017;63(1):93-98.
- Paidas MJ, Tita ATN, Macones GA, et al. Prospective, randomized, double-blind, placebo-controlled evaluation of the pharmacokinetics, safety and efficacy of recombinant antithrombin versus placebo in preterm preeclampsia. Am J Obstet Gynecol. 2020;223(5):739.e1-739.e13.
- Avidan MS, Levy JH, Scholz J, et al. A phase III, double-blind, placebo-controlled, multicenter study on the efficacy of recombinant human antithrombin in heparin-resistant patients scheduled to undergo cardiac surgery necessitating cardiopulmonary bypass. Anesthesiology. 2005;102(2):276-284.
- Edmunds T, Van Patten SM, Pollock J, et al. Transgenically produced human antithrombin: structural and functional comparison to human plasma-derived antithrombin. Blood. 1998;91(12):4561-4571.
- Adiguzel C, Iqbal O, Demir M, Fareed J. European community and US-FDA approval of recombinant human antithrombin produced in genetically altered goats. Clin Appl Thromb Hemost. 2009;15(6):645-651.
- Furuie H, Kanda H. Randomized comparison study of novel recombinant human antithrombin gamma and plasma-derived antithrombin in healthy volunteers. Clin Drug Investig. 2019;39(12):1185-1194.
- AABB, American Red Cross, America’s Blood Centers, Armed Services Blood Program. Circular of information for the use of human blood and blood components. 2021. Accessed November 11, 2024. www.aabb.org/tm/coi/Documents/coi1017.pdf
- Grover SP, Mackman N. Anticoagulant SERPINs: endogenous regulators of hemostasis and thrombosis. Front Cardiovasc Med. 2022;9:878199.
- Collen D, Tytgat GN, Claeys H, et al. Metabolism and distribution of fibrinogen. I. Fibrinogen turnover in physiological conditions in humans. Br J Haematol. 1972;22(6):681-700.
- Khawar H, Kelley W, Stevens JB, et al. Fresh frozen plasma (FFP). In: StatPearls [internet]. 2022.
- Bader SO, Marinaro XF, Stone G, Lodaya K, Spears JB, Shander A. Antithrombin concentrates may benefit cardiopulmonary bypass patients with suspected heparin resistance: a retrospective analysis of real-world data. Heliyon. 2023;9(9):e19497.
- Hyatt W, Yon JR, Haley-Andrews S. Minimizing time to plasma administration and fresh frozen plasma waste: a multimodal approach to improve massive transfusion at a level 1 trauma center. J Trauma Nurs. 2019;26(5):234-238.
- Smith TS, Evans J, Moriel K, et al. Cost of operating room time is $46.04 dollars per minute. J Ortho Business. 2022;2(4):10-13.
- Shander A, Ozawa S, Hofmann A. Activity-based costs of plasma transfusions in medical and surgical inpatients at a US hospital. Vox Sang. 2016;111(1):55-61.
- Protti A, Iapichino GE, Di Nardo M, Panigada M, Gattinoni L. Anticoagulation management and antithrombin supplementation practice during veno-venous extracorporeal membrane oxygenation: a worldwide survey. Anesthesiology. 2020;132(3):562-570.
- Zantek ND, Steiner ME, Teruya J, et al. Recommendations on monitoring and replacement of antithrombin, fibrinogen, and Von Willebrand factor in pediatric patients on extracorporeal membrane oxygenation: the Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024;25(7 suppl 1):e35-e43.
- Moront MG, Woodward MK, Essandoh MK, et al. A multicenter, randomized, double-blind, placebo-controlled trial of preoperative antithrombin supplementation in patients at risk for antithrombin deficiency after cardiac surgery. Anesth Analg. 2022;135(4):757-768.
- Murao A, Kato T, Yamane T, Honda G, Eguchi Y. Benefit profile of thrombomodulin alfa combined with antithrombin concentrate in patients with sepsis-induced disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2022;28:10760296221077096.
- Endo S, Shimazaki R; Antithrombin Gamma Study Group. An open-label, randomized, phase 3 study of the efficacy and safety of antithrombin gamma in patients with sepsis-induced disseminated intravascular coagulation syndrome. J Intensive Care. 2018;6:75.
- Kuroda H, Masuda Y. Comparison of protective effects of recombinant antithrombin gamma and plasma-derived antithrombin on sepsis-induced disseminated intravascular coagulation and multiple organ failure. Clin Appl Thromb Hemost. 2020;26:1076029620981630.
false
*You must be logged in to see results data.
- How common is inherited antithrombin deficiency (ATD) in the general population?
correct answer: a.In the general population, hereditary ATD affects approximately 0.02% to 0.2% of people but is more common in patients with VTE (1%-5%).
- With which coagulation factors does AT interact?
correct answer: c.AT acts as a natural anticoagulant by inhibiting thrombin (factor IIa) and factor Xa, as well as the procoagulant factors VIIa, IXa, XIa, XIIa, kallikrein, and plasmin.
- Which of the inherited types of ATD is the mildest?
correct answer: c.Hereditary ATD is categorized into 2 groups (types I and II) and 3 subgroups of type II. Type I carries the highest risk for VTEs. Type IIa interferes with the ability of AT to bind with its target proteases, such as thrombin, and can result in a heightened risk for VTEs. Type IIb targets the heparin-binding site, reducing the anticoagulant activity of heparin; this results in a lower risk for VTEs. Type IIc ATD (pleiotropic effect) is associated with severe VTEs, as the mutation is located in an area where it results in more than 1 functional defect.
- What clinical circumstances can result in acquired ATD?
correct answer: e.Acquired ATD manifests during heparin therapy, as used in ECMO, CPB, and continuous renal replacement therapy; as well as in states of hypercoagulability, such as sepsis, trauma, pregnancy, the postpartum period, and severe COVID-19.
- What is the recommended first step in evaluating for ATD?
correct answer: b.The Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis recommends employing an activity (functional) assay for initial testing, followed by an antigen test and adding a calculation of the activity-to-antigen ratio when the activity level is low (Figure 4).

Figure 4. Recommended approach to diagnosing AT deficiency in adults.3,11 - Which statement is correct in treatment of VTE?
correct answer: a.For the treatment of an acute VTE, the 2024 compendium of guidelines from the American College of Chest Physicians (ACCP) states that LMWH or fondaparinux are preferred over intravenously or subcutaneously administered unfractionated heparin. The direct-acting oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban are recommended over warfarin.
- What are the approved indications for AT concentrates (ATcs)?
correct answer: b.The only brand of ATc approved in the United States for the treatment of thromboembolism and prevention of perioperative and peripartum thromboembolism in patients with hereditary ATD is human plasma-derived ATc (Thrombate III). The recombinant AT product available in the United States (ATryn) is approved for the prevention—but not treatment—of perioperative and peripartum thromboembolic events in hereditary ATD.
- Which statement is correct?
correct answer: c.The hpATc product has a prolonged half-life of 2.5 to 3.8 days. The recombinant ATc product has a half-life of 11.6-17.7 hours.
- What is the origin of the recombinant ATc approved in the United States?
correct answer: b.The recombinant AT product available in the United States is produced from transgenic goats who express AT in their milk. It is contraindicated in patients with a goat or goat milk allergy, and patients need to be monitored closely for hypersensitivity reactions.
- A 20-year-old woman with long-standing intractable Crohn’s disease is referred to you for abdominoperineal resection. At assessment, a degree of ATD was noted and found to be at 70% baseline activity. What is the best course of action?
correct answer: c.Given the dual stressors of severe gastrointestinal disease and the anticipated surgery, presurgical AT replacement therapy be initiated as thromboprophylaxis. This patient should receive hpATc along with enoxaparin as LMWH 2 weeks postoperatively, along with careful monitoring for several weeks thereafter to ensure improvement of AT activity over time.
false




