1.0 AMA PRA Category 1 Credit™
Faculty
 | Paul Feuerstadt, MD, FACG, AGAF Assistant Clinical Professor of Medicine Yale University School of Medicine Attending Gastroenterologist PACT-Gastroenterology Center of Connecticut Hamden, Connecticut |
 | Anne J. Gonzales-Luna, PharmD, BCIDP Assistant Professor University of Houston College of Pharmacy Houston, Texas |
 | Robert Orenstein, DO, FACP, FIDSA Chair, Division of Infectious Diseases Associate Chair for Outpatient Practice Department of Medicine Professor of Medicine Mayo Clinic College of Medicine and Science Mayo Clinic Arizona Phoenix, Arizona |
Statement of Need/Program Overview
Clostridioides difficile infection (CDI) is associated with a profound burden on patients. Several key risk factors for recurrent CDI (rCDI) have been identified (eg, antibiotic use, advanced age, prolonged hospital length of stay). The pathophysiologic processes in rCDI, including alterations in the gut microbiota, are complex, and selecting therapy for patients with rCDI is challenging. Novel microbiota-restoration therapies for rCDI treatment are recent additions to the market; clinicians require current information on the safety and efficacy of these agents and others in late-stage development.
Goal
The goal of this activity is to educate health care professionals about the role of the microbiome in CDI and rCDI, the effects of antibiotic therapy, and the new and emerging microbiome-supportive treatment options to help support informed treatment decisions.
Intended Audiences
The intended audience for this activity comprises gastroenterologists, infectious disease clinicians, and other health care professionals interested in microbiome-based therapies for managing rCDI.
Educational Objectives
- Recognize the substantial health burdens associated with rCDI.
- Describe the pathogenesis of rCDI, including the role of alterations in the intestinal microbiota.
- Discuss the benefits and risks of antimicrobial treatment approaches for rCDI.
- Evaluate the most up-to-date clinical trial data for new and emerging microbiota-restoration therapies for rCDI treatment.
Physician Accreditation Statement
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Global Education Group (Global) and Applied Clinical Education (ACE). Global is accredited by the ACCME to provide continuing medical education for physicians.
Physician Credit Designation
Global designates this live activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Global Contact Information
For information about the accreditation of this program, please contact Global at 303-395-1782 or cme@globaleducationgroup.com.
Instructions for Obtaining Credit
To receive credit, participants must participate in the activity at www.cmezone.com/cu231 and complete and pass the post-test with a minimum score of 70%. Additionally, participants will need to complete the evaluation. CME certificates will be sent via email to those successfully completing the activity. For ACPE learners, credit will be uploaded within 60 days of completion to CPE Monitor.
Fee Information and Refund/Cancellation Policy
There is no fee for this educational activity.
Disclosures of Relevant Financial Relationships
Global adheres to the policies and guidelines, including the Standards for Integrity and Independence in Accredited CE, set forth to providers by the Accreditation Council for Continuing Medical Education (ACCME) and all other professional organizations, as applicable, stating those activities where continuing education credits are awarded must be balanced, independent, objective, and scientifically rigorous. All persons in a position to control the content of an accredited continuing education program provided by Global are required to disclose all financial relationships with any ineligible company within the past 24 months to Global. All financial relationships reported are identified as relevant and mitigated by Global in accordance with the Standards for Integrity and Independence in Accredited CE in advance of delivery of the activity to learners. The content of this activity was vetted by Global to assure objectivity and that the activity is free of commercial bias. All relevant financial relationships have been mitigated.
The faculty have the following relevant financial relationships with ineligible companies:
| Paul Feuerstadt, MD, FACG, AGAF |
|
| Anne J. Gonzales-Luna, PharmD, BCIDP |
|
| Robert Orenstein, DO, FACP, FIDSA |
|
The planners and managers have the following relevant financial relationships with ineligible companies:
- The planners and managers at Global Education Group have no relevant financial relationships to disclose.
- The planners and managers at ACE have no relevant financial relationships to disclose.
System Requirements
PC
- 1.4 GHz Intel Pentium 4 or faster processor (or equivalent)
- Windows 10, 8.1 (32-bit/64-bit); Windows 7 (32-bit/64-bit) 512 MB of RAM (1 GB recommended)
- Microsoft Internet Explorer 11 or later, Windows Edge browser, Mozilla Firefox, and Google Chrome
- For HTML Client – Google Chrome (v70.0 and above), Mozilla Firefox (v65.0 and above), and Edge (v42.0 and above)
MAC
- 1.83 GHz Intel Core Duo or faster processor 512 MB of RAM (1 GB recommended)
- MAC OS X 10.12, 10.13, and 10.14
- Mozilla Firefox, Apple Safari, Google Chrome
- For HTML Client – Google Chrome (v70.0 and above), Apple Safari (v12.0 and above), and Mozilla Firefox (v65.0 and above)
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. Global and ACE do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of any organization associated with this activity. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed in this activity should not be used by clinicians without evaluation of patient conditions and possible contraindications on dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
This activity is jointly provided by Global Education Group and Applied Clinical Education.
This activity is supported by an educational grant from Ferring Pharmaceuticals, Inc.
This activity is distributed by Gastroenterology and Endoscopy News, Infectious Disease Special Edition, Pharmacy Practice News, and CMEZone.com.
false
Clostridioides difficile is a gram-positive, spore-forming bacterium that can live in the human intestine but causes inflammation and disruptive gastrointestinal (GI) symptoms if its population is not kept under control by the surrounding microbial ecosystem. C. difficile infection (CDI) can range from a mild diarrheal illness to a life-threatening, persistent, or recurrent condition that poses a significant challenge to affected patients and health care systems. According to the US Centers for Disease Control and Prevention (CDC), approximately 500,000 cases of CDI were reported in the United States in 2017, including in approximately 223,900 hospitalized patients, and CDI resulted in an estimated 12,800 deaths.1 In 2020, CDC surveillance data indicated that the crude overall incidence of CDI was 101.3 cases per 100,000 persons.2 Although the number of infections appears to be decreasing overall, CDI often recurs and remains challenging to treat.3

The gut microbiota (also referred to as the microbiome) plays a central role in preventing initial and recurrent CDI (rCDI). Sustained effective treatment of CDI requires understanding this role and how various CDI treatments affect gut microbial diversity and defenses against C. difficile. For example, exposure to antibiotics for other infections may adversely affect host defenses against C. difficile. Even the targeted antimicrobials used to treat CDI can lead to CDI recurrence by further altering the colonic microbiota. Health care providers should carefully consider treatment approaches aimed at eradicating C. difficile while minimizing the risk for CDI recurrence.
This presentation discusses approaches to harnessing the microbiome to combat rCDI, including a review of the role of the microbiome in rCDI, considerations for antibiotic selection for rCDI, and a review of new and emerging microbiota-based therapies for preventing rCDI. A case study is incorporated at different stages of the presentation to emphasize key points.
Burden of CDI

Historically, CDI was more commonly seen in the health care setting than in the community. Today, due to health care system interventions, such as antimicrobial stewardship and improved infection prevention measures, rates have decreased in many hospitals, and there has been a shift toward more community-onset cases. Recent data suggest that CDI incidence is slightly higher in the community than in hospitals, with 51.2 vs 50.1 cases per 100,000 persons, respectively. Community cases may also be responsible for seeding CDI within the health care environment.2 Patients with CDI typically receive treatment with nonabsorbed oral antibiotics such as vancomycin or fidaxomicin. Most patients with an initial bout of CDI will improve with this treatment; however, a significant number of cases recur when treatment stops or with concomitant antibiotic therapy, which then propagates the cycle of CDI. Multiple rounds of recurrence can trap patients in a vicious cycle of infection that is significantly detrimental to their quality of life and is often difficult to escape.
CDI causes substantial clinical, social, and economic burdens to patients, health care professionals, and hospital systems (Figure 1). The vegetative form of C. difficile produces toxins that elicit an inflammatory response, and that can lead to severe clinical consequences, including sepsis, toxic megacolon, dehydration, acute kidney injury, and intestinal perforation. These may then lead to colectomy, prolonged hospitalization, need for critical care, and mortality.4 The symptoms of CDI, including (but not limited to) severe diarrhea, incontinence, urgency, painful cramping, nausea, and vomiting, have significant negative effects on patient quality of life.4 Individuals affected by CDI may experience negative social effects stemming from their symptoms, including depression, anxiety, post-traumatic stress disorder, social isolation, absenteeism, loss of productivity, fear of repeat infections, and fear of infecting others.4 Some may resist future antibiotic treatment for fear of re-developing CDI.5 CDI also has significant economic consequences related to inpatient, emergency department, pharmacy, out-of-pocket, and reimbursement costs; increased length of stay; hospital readmission; and reimbursement penalties.4 These adverse individual and societal consequences underscore the need for effective and durable CDI therapies.
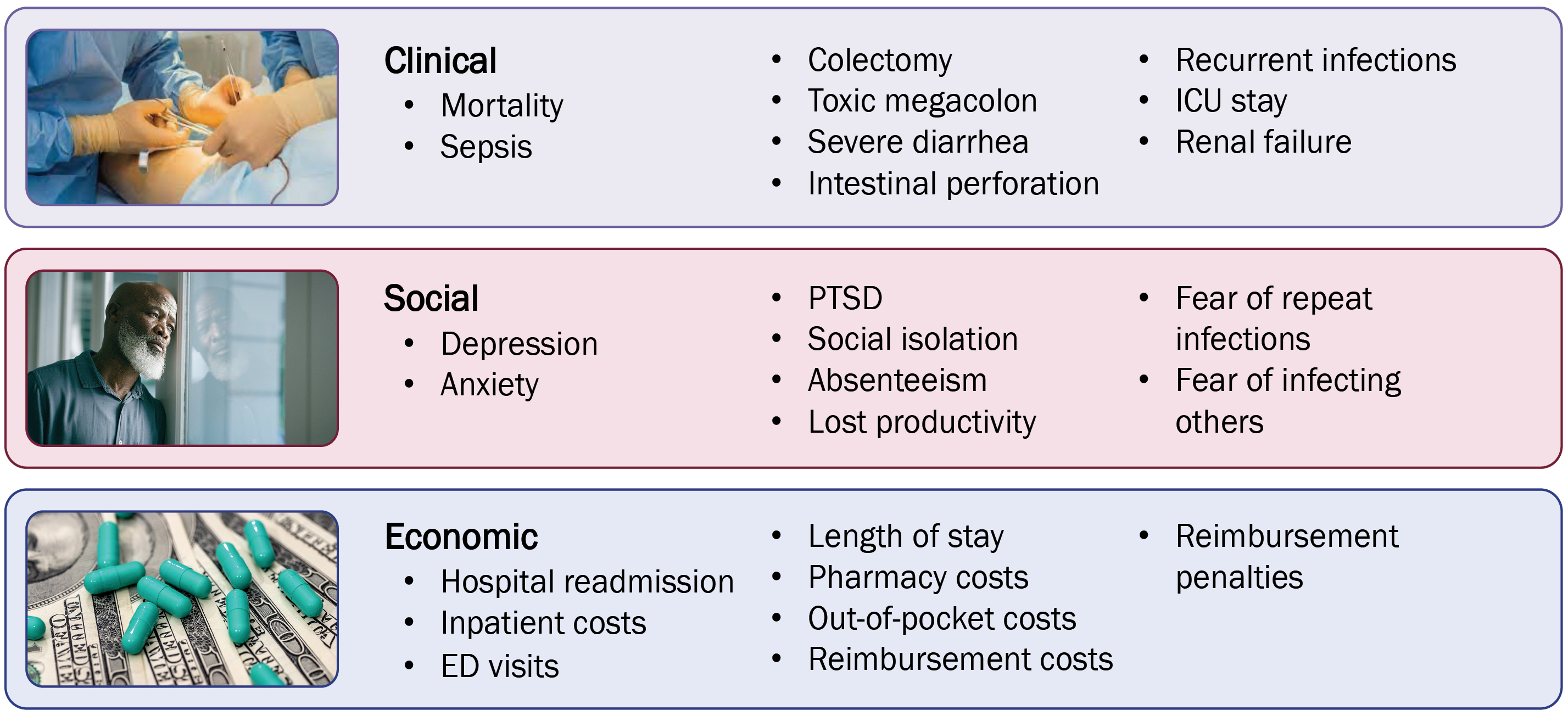
Risk Factors for CDI
The most common risk factors for an initial episode of CDI include recent hospitalization, antibiotic exposure, and older age.6 As discussed in later sections, antibiotic exposure is a key modifiable risk factor for CDI that disrupts the microbial defenses of the host gut microbiome, leading to a 2- to 16-fold increase in the risk for developing symptomatic infection.6,7 According to the CDC, 61% of CDI cases are associated with antibiotic use for other indications within the 12 weeks before C. difficile detection in stool.2 Advanced age (>65 years) nearly triples the risk for CDI, but younger patients can also be affected and should be assessed for CDI if experiencing symptoms.6-8 Traditionally thought of as a hospital-acquired illness, CDI risk remains linked to hospitalization as well as to residence in a skilled nursing facility.6,7
Additional risk factors for CDI include being female and/or Caucasian and having contact with carriers or individuals with active infection.6 Individuals with CDI often have multiple comorbidities: The Charlson Comorbidity Index is ≥2 in 40% of cases.9 More recently, undergoing gastric acid suppression via proton pump inhibitor (PPI) use has been linked to CDI, as it is suspected that gastric acid has a protective effect against C. difficile colonization.6
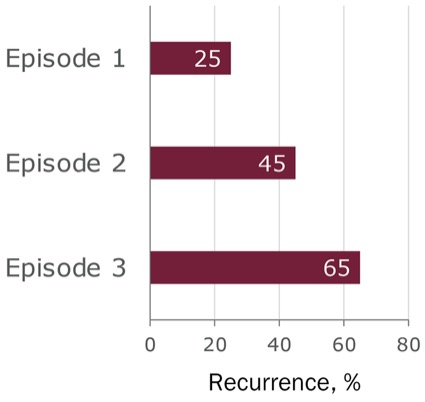
Perhaps the most concerning risk factor for recurrence for providers and patients is a previous episode of CDI. Preventing recurrence has become a major focus of treatment because it can lead to a cycle of reinfection that makes recovery increasingly difficult. For patients with a single previous episode of CDI, the risk for recurrent infection is 20% to 30%, and the rate of recurrence increases with each episode up to 65% (Figure 2).9,10 In addition to previous episodes of CDI, risk factors for rCDI include age 65 or older, history of severe CDI, increasing peripheral leukocyte count, hypoalbuminemia, fever, presence of comorbidities, inflammatory bowel disease (IBD), ongoing or recurrent antibiotic exposure, decreased serum anti-toxin A immunoglobulin (Ig)G, and possibly the use of PPIs.6 Understanding these risk factors can help clinicians make treatment decisions that lead to a lasting cure.
Role of the Microbiome
The gut microbiota and microbiome play major roles in an individual’s susceptibility to CDI and rCDI. In a healthy human, the intestinal microbiota are diverse, with multiple species of organisms playing different and redundant roles in maintaining hemostasis, immune function, epithelial barrier integrity, metabolism, and energy production.11 Although the majority of our understanding is focused on the bacterial microbiome, we are starting to understand the other key organisms that affect the functions of the microbiome, including fungi, viruses, and archaea.11 Initially shaped during birth and with early diet and exposures, the microbiome is a dynamic and evolving entity that adapts and changes with environmental factors, including rapid changes between birth and age 3.11 Eventually, the microbiome reaches a level of stability between ages 30 and 70 and decreases in diversity in older age.10 This microbial diversity is critical to many processes in human biology and serves as a key line of defense against intestinal pathogens.11
In human health, the GI microbiome makes beneficial contributions to a range of biological processes. Microorganisms support healthy digestive function and metabolism, for example, by metabolizing carbohydrates, proteins, and lipids and producing vitamins D, K, B12, and other nutritional factors.11 Certain species also affect the metabolism of toxins and pharmaceutical agents and can affect how patients metabolize medications.11 The microbiome is capable of immunomodulation via the production of metabolites that affect immune function and immune cell signaling.11 A healthy gut microbiome also influences epithelial barrier function and helps resist colonization by harmful organisms and exogenous pathogens.12
The various microbial populations residing in the gut perform a range of beneficial functions that control populations of harmful microorganisms, including C. difficile.12 An insult to the delicate balance of the gut microbial environment can cause a decline in beneficial and commensal species populations and allow the overgrowth of pathogens, such as C. difficile.7 Resident microbial species produce agents that inhibit the growth of other bacteria; for example, bacteria in the gut produce immune-stimulating factors that influence the production of primary and secondary bile acids, which may be protective against C. difficile spores and CDI, respectively.13 Resistance to colonization is a major factor associated with the development of CDI and rCDI; indeed, many risk factors associated with CDI affect gut microbial diversity—particularly exposure to antimicrobials. Understanding the role of microbial diversity is essential to making CDI treatment decisions that will support, preserve, and restore the body’s natural defense.
Negative Effects of Dysbiosis
Dysbiosis describes a disturbance of the healthy microbiome that results in a detrimental decrease in the diversity of the populations and functions of the microorganisms within the GI tract.13 In particular, dysbiosis is marked by a reduction in species from the phyla Bacteroidetes and Firmicutes (the major constituents of the GI microbiome), as well as a proliferation of Proteobacteria (eg, Escherichia coli and other gram-negative bacteria).13 Triggers of dysbiosis may include factors such as antibiotics, stress, diet, medications, and hygiene.14 These perturbations disrupt the balance of the gut microbiome. The resulting dysbiosis has wide-ranging negative effects on the human body and, in addition to CDI, is thought to be associated with various types of cancer, IBD, irritable bowel syndrome (IBS), obesity, type 2 diabetes, rheumatoid arthritis, and autism.13
Dysbiosis lowers host defenses against colonization by harmful bacteria and favors the growth of C. difficile.13 Uninhibited C. difficile growth can damage the intestinal epithelium on macro- and microscopic levels.15 C. difficile can alter inflammatory cytokine signaling and trigger an inflammatory reaction within the GI tract. This loss of key metabolic actions (eg, secondary bile acid synthesis) that normally enhance gut immunity and protect epithelial integrity can make an individual susceptible to CDI and other infections. Disruption of the delicate balance of bile acids via insult to bile acid synthesis pathways can fuel the growth of C. difficile: Primary bile acids encourage the germination of C. difficile spores, and loss of the protective microbiota prevents conversion of primary bile acids to secondary bile acids that inhibit the vegetative phase that releases toxins causing symptoms.13,16 In addition, the synthesis of secondary bile acids (deoxycholic and lithocholic acid), which inhibit the growth of C. difficile, is hindered by the loss of other protective Clostridia species.13 This disruption in bile acid synthesis results in environmental changes that favor the sporulation and subsequent growth of C. difficile. Effectively treating CDI and preventing future recurrence depends on repairing dysbiosis to restore healthy microbial diversity.
Factors Contributing to Dysbiosis
Multiple factors can insult the balance of the microbiota and lead to dysbiosis, which is reflected in the risk factors for CDI. Antimicrobial treatment quickly decreases GI species richness, which may not recover through natural regrowth after treatment cessation (Figure 3).17 PPIs reduce gastric acid secretion and lay the foundation for colonization by C. difficile by lowering the pH of the digestive system, which negatively affects commensal microbial species, including Ruminococcus and Dorea, indirectly stimulates oral microbes, and increases the population of harmful bacterial species.18-21 Long-term PPI use affects survival and induces the migration of multiple bacteria along the GI tract, further increasing the risk for gut dysbiosis.21 Metabolic changes resulting from PPI use may also expose sugars that support the growth of C. difficile.
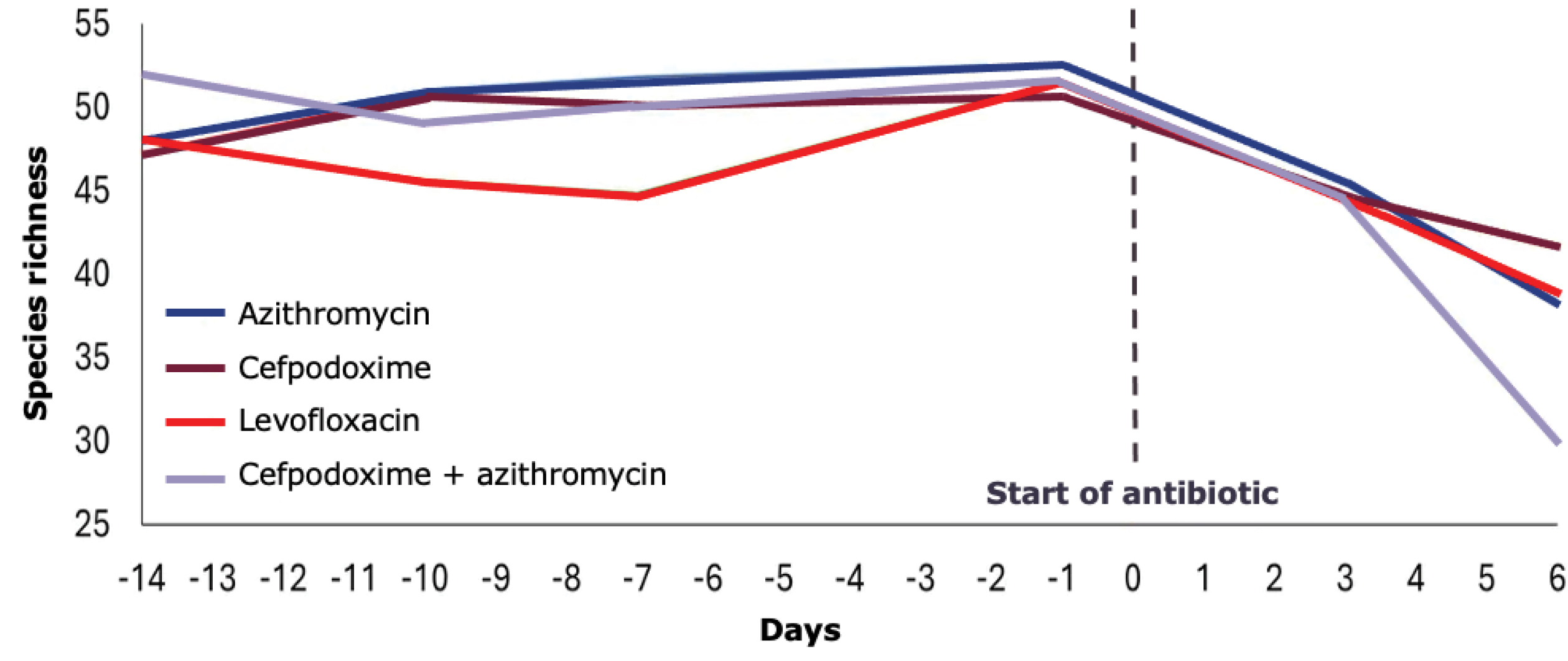
Treatment for CDI can also exacerbate a dysbiotic state. The antibiotic vancomycin is the most common treatment for CDI. It induces consistent changes in the human intestinal microbiota. Although some patients recover quickly from the negative effects of vancomycin exposure, others may not. In one study, 21 patients with CDI were randomly assigned to treatment with oral vancomycin for 2 weeks, followed by an immunosuppressant, methotrexate, beginning 6 weeks after vancomycin cessation, or to oral methotrexate only. Patients in the vancomycin group experienced significant declines in gut microbial diversity, from which few recovered 22 weeks later (Figure 4).22

Importantly, there is a recovery period after the cessation of antimicrobial treatment for CDI, when the microbiota is restoring the deficiencies, which can leave them vulnerable to recolonization before they can fully restore microbial diversity. For vancomycin and metronidazole treatment, the highest risk window of vulnerability extends for approximately 3 weeks after treatment cessation.23 Patients who have experienced multiple episodes of CDI endure cumulative damage to gut microbial diversity that increases the risk for subsequent infection, underscoring the importance of preventing CDI recurrence in the early stages.
Harnessing the Microbiome to Predict CDI Recurrence
Given the effect of the gut microbiome on human metabolism, it follows that metabolomics, the metabolic output from the microbiota, can predict recurrence. A study of human stool samples identified key metabolic changes that increased the risk for rCDI, including host inflammation or intestinal injury, a lack of microbial deconjugation (bile acid) activity, and alterations in immune and inflammatory abilities.24 The longitudinal, multicenter study was a multi-omics analysis of serial stool samples collected from 53 patients with CDI.24 Among these patients, the rate of recovery from dysbiosis was slower in those with recurrence than in those without. A specific metabolic profile (increased sphingolipids, phospholipids, and sphingomyelins) predicted recurrence with an area under the receiver operating characteristic curve of 0.77 (95% confidence interval [CI], 0.71-0.86) at 1 week and 0.77 (95% CI, 0.69-0.85), at 2 weeks.24 These data emphasize the close interplay between gut microbial dynamics and CDI.

Case Study: Lorraine
Presentation
Lorraine is a 60-year-old woman who presents in May 2023 with sudden onset of 6 to 8 liquid bowel movements per day. She complains of occasional sweats and diffuse cramping abdominal pain (3/10) that is relieved with bowel movement. She has no recent travel, sick contacts, or antimicrobial exposure. Her medical history includes hypertension, diabetes, gastroesophageal reflux disease, and a previous CDI in March 2023. She has a surgical history of appendectomy. Lorraine’s initial blood work shows a white blood cell (WBC) count of 11,000×103/mL and a creatinine level of 1.1 mg/dL. Lorraine is further assessed with a polymerase chain reaction (PCR) test and diagnosed with CDI.
It is important to note that from a microbiome perspective, deficiencies of Bacteroidetes and Firmicutes are the most likely disruptions to have led to Lorraine’s presentation of CDI.
“The bottom line here is the more [CDI] episodes you have, the less diverse your gut microbiota. And of course, if you’re getting treated for every one of these episodes, you continue to reduce the diversity of the gut microbiota, making it unlikely that you’re going to be able to clear the infection after you’ve had multiple episodes.”

Despite its known contribution to dysbiosis, the long-standing treatment paradigm for CDI remains centered on the use of antibiotics for C. difficile eradication. This will not change anytime soon. Over time, researchers and clinicians have gained a greater appreciation of the effect of these antibiotic treatments on the gut microbial environment and the implications for patients with CDI. In the search for more effective treatments, clinical trial outcomes have evolved to reflect this improved understanding, particularly regarding the risk for CDI recurrence. Phase 3 clinical trials have shifted from primary end points of initial cure following antimicrobial therapy, in which treatment aimed to cure the primary infection, to sustained clinical response (measured at least 30 days after treatment completion), reflecting treatment that stops the cycle of recurrence.25 More than 85% of patients initially respond to standard-of-care (SOC) antibiotic treatment (vancomycin or metronidazole), with up to 80% maintaining a sustained response.26 However, up to 25% of patients will have recurrence of initial infection, and as many as 65% will have 3 or more recurrences.9,10
With regard to their effect on gut microbial diversity, not all antimicrobials are created equally.27 Careful selection of antibiotic therapies can help providers increase their patients’ chances of achieving sustained response and avoiding CDI recurrence.27 It is important to understand that different antibiotic therapies have varying effects on microbial diversity through their effects on biliary excretion, the types of organisms they target, and the accumulation of exposure over time.27-30 The abundance of antibiotic-resistant and antibiotic-susceptible organisms in the gut is also affected.27 Understanding the effect of available therapeutic options for CDI on the microbiota can help make more informed and effective treatment decisions.
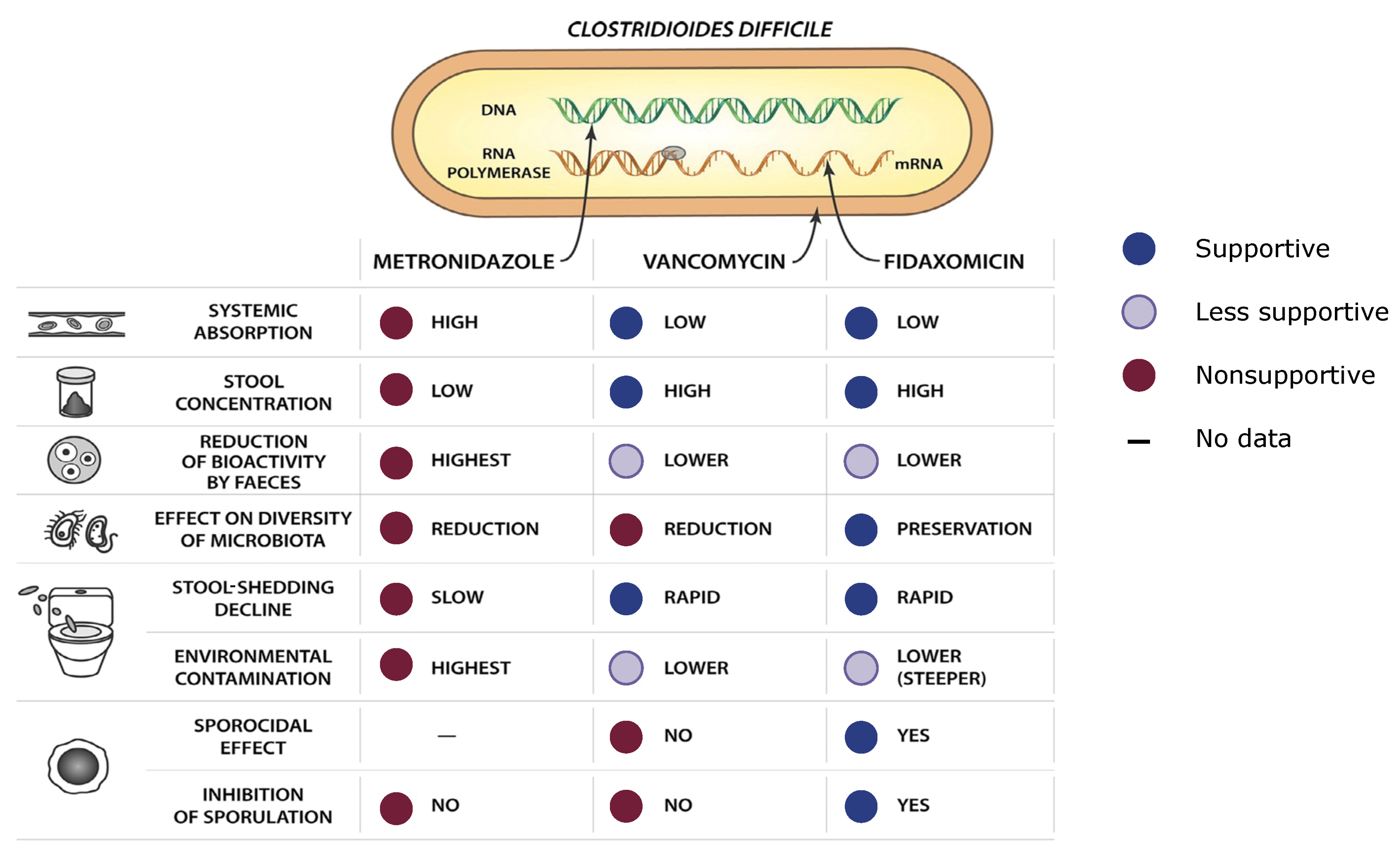
Selecting Among Antibiotics
The antibiotics most commonly used to treat CDI vary in their effect on the gut microbiome and, in turn, the CDI disease course (Figure 5).27 Metronidazole, for example, has several features that make it a less ideal selection for CDI treatment than other agents: It exhibits high systemic absorption and low stool concentration (vancomycin and fidaxomicin show low systemic absorption and high stool concentration), and some studies indicate that nitroreductase-producing enterococci that are present in the gut could deactivate or reduce metronidazole activity.27 Fidaxomicin, on the other hand, has features that may make it a better choice for CDI treatment. It spares more of the host microbiome compared with vancomycin and metronidazole.27 Fidaxomicin and vancomycin more rapidly decrease C. difficile shedding in stool, which decreases environmental contamination and likely transmission.27 The mechanism of action of fidaxomicin is also more favorable compared with the other 2 agents as, in addition to its activity against the C. difficile vegetative phase, it can inhibit C. difficile spore germination, showing an antimicrobial activity against those spores.27
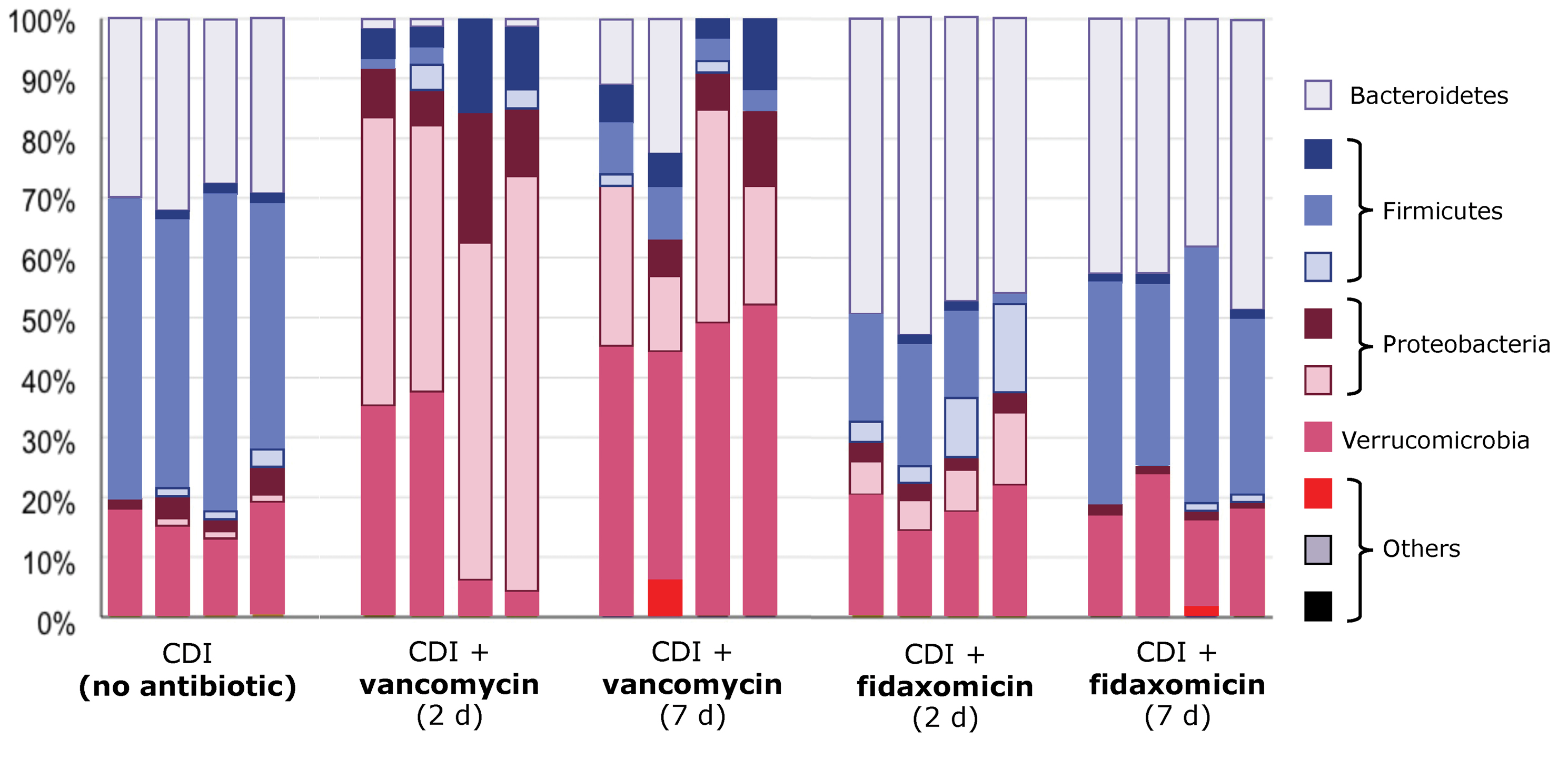
Antibiotic therapy for CDI effectively addresses active infection in most cases, but off-target effects of treatment exacerbate dysbiosis. In a study performed in mice with CDI, the relative abundance of bacterial species was altered drastically in those treated with vancomycin compared with untreated mice and mice treated with fidaxomicin (Figure 6).31 As discussed in the previous section, this insult to microbial diversity fuels dysbiosis and creates an environment that favors the recurrence of C. difficile.
Data from human clinical studies show how different antibiotic treatments for CDI affect the risk for recurrence (Figure 7). Three phase 3 trials and 1 phase 4 trial have assessed clinical cure rates and recurrence for patients with CDI treated with vancomycin and fidaxomicin.32-35 Across all trials, no difference in clinical cure rates was observed between patients given vancomycin or fidaxomicin, indicating that both treatments are equally effective for initial response. However, in 3 of 4 of those trials, recurrence rates were significantly lower in patients treated with fidaxomicin than vancomycin, in agreement with many of the previously discussed drug characteristics.32-35

Taken together, these data suggest that fidaxomicin is preferable as a treatment for CDI due to its ability to reach high concentrations in the stool with minimal effect on the host microbiome and its activity against C. difficile spores. However, fidaxomicin can be difficult for some patients to obtain, and vancomycin remains the most common treatment.2
CDI Treatment Guidelines
In 2021, the American College of Gastroenterology (ACG) released clinical guidelines making recommendations about diagnosing and treating CDI (Figure 8).36 The ACG recommends treatment approaches informed by the patient’s CDI history and whether the current infection is the patient’s initial episode, first recurrence, or second or greater recurrent episode.36 Treatment generally becomes more aggressive with additional recurrences. For a first episode, treatment options are stratified based on the severity of infection.36

- For non-severe infection, defined by a WBC <15,000/mL3 and creatinine <1.5 mg/dL, the ACG recommends treatment with oral vancomycin (125 mg 4 times daily for 10 days; strong recommendation) or oral fidaxomicin (200 mg twice daily for 10 days; strong recommendation). Oral metronidazole (500 mg 3 times daily for 10 days; strong recommendation) is listed as an additional option for low-risk patients (young patients, outpatient setting).
- For patients with severe disease (eg, WBC >15,000/mL3 or creatinine >1.5 mg/dL), oral vancomycin (125 mg 4 times daily for 10 days; strong recommendation) or fidaxomicin (200 mg twice daily for 10 days; conditional recommendation) are recommended.
Upon first recurrence, the ACG recommends tapering/pulsed-dose vancomycin after an initial course of fidaxomicin, vancomycin, or metronidazole (strong recommendation).36 As an alternative, if vancomycin or metronidazole was given for the initial episode, the patient can be treated with fidaxomicin 200 mg twice daily for 10 days (strong recommendation).36 Tapered and pulsed regimens use repeating cycles of antibiotic-free periods and pulses of antibiotics that correspond to the different stages of the C. difficile life cycle. The antibiotic-free periods allow the C. difficile spores to germinate (and allow the host microbiome time to recolonize), and the subsequent antibiotic pulse kills off the newly germinated vegetative C. difficile cells.37 Vancomycin is typically used for tapered and pulsed regimens, starting at a 125 mg dose and decreasing the dose by 25% to 50% every 1 to 2 weeks with no skipped days, then pulsing at the 125 mg dose, skipping 1 to 2 days, for 2 to 4 weeks. Treatment with tapered/pulsed vancomycin has been shown to lower recurrence rates compared with non-tapered/pulsed treatment approaches.37 Fidaxomicin can also be used for tapered and pulsed dosing; data from the EXTEND trial, a phase 3 trial performed in hospitalized adults older than age 60, illustrates the efficacy of fidaxomicin in this regimen.33 In EXTEND, prolonged dosing with fidaxomicin (200 mg oral tablets twice daily on days 1 to 5, then 1 dose on alternate days for days 7 to 25) was superior to vancomycin for sustained cure of CDI assessed 90 days after the end of treatment. Rates of sustained clinical cure were 70% in the fidaxomicin group compared with 59% in those who received vancomycin (odds ratio [OR], 1.62; 95% CI, 1.04-2.54; P=0.030).33 The difference in outcome was driven by the significantly lower rates of recurrence among patients given fidaxomicin.33
For additional recurrences, the ACG recommends a course of antimicrobials followed by FMT via colonoscopy or capsules (strong recommendation) or by enema if other approaches are not possible (conditional recommendation). Long-term, suppressive oral vancomycin is recommended if the patient relapses after FMT, is not a candidate for FMT, or is continually or frequently exposed to antibiotics (conditional recommendation).36
Incorporating Bezlotoxumab
Bezlotoxumab is a fully humanized IgG1 monoclonal antibody used as an adjunctive therapy to prevent rCDI through binding to toxin B, a potent toxin produced by C. difficile that causes mucosal injury, acute inflammation, and diarrhea.36 Interaction between bezlotoxumab and toxin B prevents the toxin from entering the GI system and mitigates tissue damage.36 Across all recurrence categories, bezlotoxumab is recommended by the ACG for the prevention of recurrence in patients at high risk for future recurrence (risk factors include age 65 years or older, a second CDI episode in the past 6 months, immunocompromised status, or severe CDI).36
In the phase 3 MODIFY I and MODIFY II trials, patients treated with bezlotoxumab vs placebo showed significantly higher rates of sustained cure (pooled analysis: 63.5% vs 53.7%; adjusted difference,
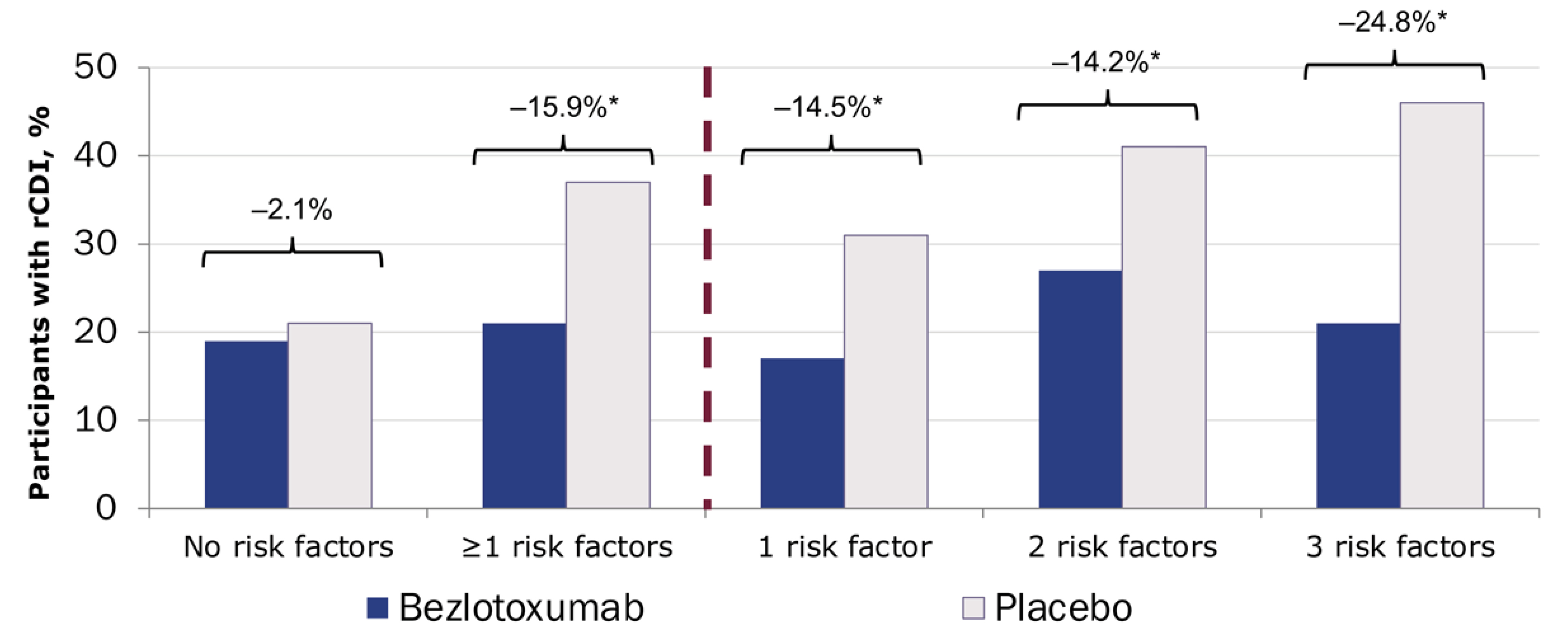
Notably, only 4% of patients in these trials received fidaxomicin as primary therapy; most were treated with metronidazole (47%) or vancomycin (48%).38 In a post hoc analysis, only patients with risk factors for recurrence derived benefit from bezlotoxumab treatment (Figure 9; 20.9% with bezlotoxumab vs 18.8% with placebo), and benefit increased with the number of risk factors.39 Based on these data, treatment with bezlotoxumab should be reserved for those at high risk for recurrence, in accordance with the FDA indication.

Bezlotoxumab is administered as an IV infusion in a single 10 mg/kg dose.36 The half-life of bezlotoxumab is 19 days, so it can be given at any time during active CDI antibiotic treatment.36 This agent should be used with caution in patients with congestive heart failure; clinical trials indicated an increased risk for heart failure (HF) among patients with underlying HF (2% vs 1% for those with and without underlying HF, respectively).36 The ACG does not recommend the use of bezlotoxumab in patients with a history of HF.36 The risks and benefits of bezlotoxumab treatment should be weighed before treating a patient with HF or other cardiovascular comorbidities.
Optimizing Antibiotics for CDI
Clinicians can adopt strategies to obtain the greatest benefit from antibiotic treatment while minimizing the risk for recurrence. The following list outlines clinical approaches that leverage the understanding of the microbiome to increase the likelihood of sustained cure for patients with CDI.
- Minimize unnecessary antibiotic exposure, including exposure to CDI-directed antibiotics.
- Use minimal necessary antimicrobial treatment duration to achieve efficacy (ie, 10 vs 14 days of treatment)
- Avoid combination therapy unless treating fulminant disease
- Think hard about using vancomycin prophylaxis. Although some patients can benefit from this approach, clinicians should consider the overall effect of vancomycin overtreatment on the microbiome and weigh the risks and benefits on a per-patient basis
- Use the most narrow-spectrum antibiotic as early as possible to preserve host microbiota (eg, fidaxomicin vs vancomycin).
- Narrow-spectrum antibiotics have fewer off-target effects and minimize effects on gut biodiversity
- Increases likelihood of sustained clinical cure
- The advantage of narrow-spectrum antibiotics is lessened when used for later treatment courses or after broad-spectrum antibiotics
Clinicians should remember that antibiotic treatments for CDI have important overlaps with the antibiotic exposures that cause CDI. All CDI-directed antibiotics cause some degree of collateral damage to the host microbiota, furthering dysbiosis. More narrow-spectrum CDI antibiotics minimize these disruptions and preserve more of the remaining host microbiota. However, the current treatment paradigm does not restore microbiota diversity; it relies on the natural regrowth of those deficiencies. Following these strategies and using a microbiome-informed approach to CDI treatment can help patients fully recover from infection and avoid the cycle of CDI recurrence.

Case Study: Lorraine
Treatment
Lorraine is treated with oral vancomycin 125 mg 4 times daily for 10 days and responds.
Subsequent symptoms
Four weeks later, Lorraine experiences abdominal pain with 6 to 9 liquid stools per day. She calls her primary care physician and is referred to your office for further assessment. Her blood work shows a WBC count of 9000×103/mL and a creatinine level of 0.9 mg/dL.
Commentary
Several treatment options are available for Lorraine. Because she has already failed a standard course of vancomycin, re-treatment with SOC vancomycin is not the best choice. Adding rifaximin 550 mg to the standard vancomycin treatment is also not ideal, given the clinical data in support of rifaximin being from smaller trials and in the context of previous exposure to metronidazole, not vancomycin.
Depending on Lorraine’s insurance, fidaxomicin treatment, according to the EXTEND trial regimen, may be the preferred treatment based on the up-front efficacy (80%) and low rate of recurrence (6%). However, supply issues may hinder access to fidaxomicin. If fidaxomicin is not accessible, a vancomycin taper-pulse regimen could be used instead. A recent meta-analysis showed an efficacy of 83% with vancomycin taper-pulse.40 Lorraine is treated with oral vancomycin 125 mg 4 times daily for 10 days and responds.
“If you take a step back, I think there’s some hypocrisy built into this paradigm we follow. This dysbiosis that is almost necessarily required for CDI to develop is most classically actually caused by antibiotics. And of course, we paradoxically then treat that infection with more antibiotics, hoping for an initial response. Historically, this has occurred in 80% to 90% of patients with standard-of-care antibiotics, so antibiotics have always worked pretty well for obtaining initial response. Around 80% of those then go on to have a sustained response, but also, somewhere between a fourth and fifth of these patients historically go on to have a recurrent infection and get stuck in this cycle of recurrence. And once you’re in that cycle, it’s even harder to get out. But not all antibiotics are created equally.”

Multimodal treatment is an emerging therapeutic strategy for patients with rCDI. Several new and emerging therapies are showing promise for use in the adjuvant treatment setting. Adjuvant treatment leverages the C. difficile life cycle, which moves through 2 stages: the vegetative state, in which C. difficile releases toxins and causes symptoms, and the spore phase, in which C. difficile does not cause disease but can persist in a more dormant state for longer periods. The traditional treatments of fidaxomicin, vancomycin, and metronidazole all address the vegetative state of infection, where they effectively kill the active infection, address symptoms, and help patients feel better. However, for some patients treated with SOC therapy, the microbiome is unable to recover quickly enough after treatment cessation, and recurrence occurs. Several new treatments are designed to support the microbiome during this recovery stage to prevent recurrence.
The goal of microbiome-preventative treatment for CDI is to help restore the gut microbial diversity to harness its protective functions against C. difficile recurrence. The treatment with the most enthusiasm surrounding it is FMT, recommended by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America (IDSA/SHEA), and the ACG to prevent further recurrence of rCDI (Table 1). FMT is positioned in both guidelines for patients with a second recurrence and beyond (eg, third episode).36,41 The ACG guidelines provide additional information on the indications for FMT, recommending an additional course of antibiotics followed by repeat FMT for patients who develop recurrence within 8 weeks of initial treatment.36
| Table 1. FMT Recommendations for CDI From the IDSA/SHEA and ACG36,4 | |||
| Group | Recurrence | Recommendation/ Opinion | Strength |
|---|---|---|---|
| ACG36 | ≥2 recurrences (ie, 3 episodes) | FMT to prevent further recurrence |
|
| Recurrence in ≤8 wk of initial FMT | Repeat FMT |
|
|
| IDSA, SHEA41 | ≥2 recurrences (ie, 3 episodes): should be tried | Appropriate antibiotic treatment before offering FMT | n/a |
| ACG, American College of Gastroenterology; CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation; IDSA, Infectious Diseases Society of America; SHEA, Society for Healthcare Epidemiology of America. | |||
FMT Clinical Trials
The results of several foundational clinical trials have generated enthusiasm for FMT as a preventative treatment for recurrent CDI. In 2013, a prospective, randomized, open-label trial compared FMT with vancomycin and bowel lavage and vancomycin alone in 43 patients with recurrent CDI after at least 1 course of SOC antibiotic treatment (vancomycin or metronidazole).42 The study was stopped after interim analyses showed an overall efficacy of CDI resolution of 81% in the FMT arm, compared with 23% and 31% in the vancomycin and bowel lavage and vancomycin-alone arms, respectively (Figure 10). Many mostly retrospective studies ensued. As summarized in a 2017 meta-analysis, across 37 total studies (7 randomized controlled trials and 30 case series), a rate of CDI resolution was reported to be 92% (95% CI, 89%-94%) after FMT.43
Not all patients respond similarly to FMT. In a prospective study of data from the American Gastroenterological Association (AGA) Institute FMT National Registry (a multicenter registry of North American FMT recipients), 1-month efficacy rates were 90% (95% CI, 85%-93%) among patients with data available. However, many patients were lost to follow-up, resulting in an overall efficacy rate of 86% (95% CI, 82%-90%) in the intent-to-treat population.44 Among responding patients, the 6-month recurrence rate was 4%.44 In another study, data from the OpenBiome stool bank revealed an overall 8-week clinical response rate of 78% (95% CI, 77%-80%), and the response rate for lower administration, deemed the most effective delivery method, was 81% (95% CI, 80%-82%).45 A recent meta-analysis of 19 prospective clinical trials assessing the prevention of CDI recurrence via FMT found a weighted pooled clinical cure rate of 78% (95% CI, 71%-85%) among patients treated with a single FMT.46 In analyses only including trials with a control arm, the weighted pooled rate of clinical cure was 72% (95% CI, 60%-82%).46 These data indicate a high response rate but one with room for continued improvement.

Clinical Considerations for Microbiota Replacement Therapy
FMT belongs to a class of treatments collectively referred to as microbiota replacement therapies (MRT). In addition to FMT, MRT includes live biotherapeutic products (LBPs), biological treatments made up of live microorganisms designed to prevent, treat, or cure a disease or condition. Administration of FMT and LBP is performed with the same goal of recolonizing a healthy, diverse gut microbiome. However, there are important differences among the treatments.
Broadly speaking, producing an LBP is a more stringent and controlled process than collecting donor stool for FMT. Compared with FMT, which requires standard screening for factors such as viruses, parasites, and other infectious diseases, LBP involves much broader donor screening, including donor history, medications, and the risk for transmission of infection. In addition, although FMT does not guarantee that the donor has the appropriate Bacteroidetes and Firmicutes species required to prevent CDI recurrence, LBPs have proprietary elements and consistent consortia to ensure the provision of the necessary microorganisms to support recolonization after CDI. LBPs are also produced according to Good Manufacturing Practices, with stronger support from clinical trial data and high-quality safety data reported. Ease of access may also differ between the 2 treatments. FMT may be easy for providers with Investigational New Drug (IND) permission from the FDA; providers without IND access, which is the overwhelming majority of those in clinical practice, may or may not have easy access to LBPs, depending on insurance coverage. Several new LBPs are being assessed as “add-on” treatments for CDI and given these benefits, and it is preferable to FMT.
Considerations for LBP Clinical Trials
Clinicians should pay close attention to several CDI-specific factors when evaluating data from LBP clinical trials. In particular, the baseline number of episodes of CDI in the study population is important to consider as it will be more difficult to recover the microbial population in patients with multiple recurrences, and patients treated earlier in the disease course may fare better given a less dysbiotic state.9 In addition, many trials use PCR to diagnose CDI for trial enrollment; however, PCR is notorious for over-diagnosing CDI. For a more reliable diagnosis, PCR should be combined with other clinical considerations (eg, >3 liquid bowel movements in a 24-hour period, stools that take the shape of the specimen collection kit, and lack of laxative treatment at the time of stool sample collection); solitary PCR may result in patients with other diagnoses (eg, post-infection IBS) receiving incorrect treatment for the disorder and thus not responding.
Duration of treatment before MRT is another key consideration; although the optimal length of SOC therapy is not known, a minimum of 10 days is believed to be required; longer is not considered to be better necessarily. The goal of CDI treatment is to sufficiently suppress the vegetative phase with antimicrobial therapy to control symptoms, allowing the body to subsequently replenish the microbiota and suppress the spore phase to prevent a recurrence. To this end, MRT is an adjuvant treatment and should always be administered after SOC antibiotic therapy.
Finally, the incorporation of a washout period is critical to the success of MRT, as residual antimicrobials will negatively affect the transplanted microorganisms if sufficient time is not provided to move the previous treatments through the patient’s system. The goals of the washout period are to clear as much antimicrobial as possible without allowing too much time for C. difficile to reconvert to the vegetative phase and cause a recurrence. The optimal timing for a washout period is unclear, and clinical trials may use varying approaches. The following sections describe new and emerging LBP products used and investigated as CDI preventative treatments. Consideration of the elements outlined above is key to appropriately evaluating the outcomes of these clinical trials.
Fecal Microbiota, Live-jslm
The first FDA-approved LBP is fecal microbiota, live-jslm (Rebyota). This single-dose, microbiota-based LBP comprises 150 mL of therapeutic material, containing 107 microbes/mL or 15×108 microbes per treatment.47 After SOC antimicrobial treatment and a washout period of 24 to 72 hours, it is administered as a single rectal instillation.47 Before its approval, this therapy was identified by different names in the literature, including RBX2660, but we refer to it as RBL in this document.
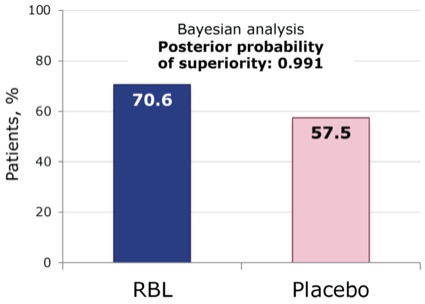
Supporting data for the use of RBL for the prevention of recurrence of CDI were collected in the pivotal phase 3 PUNCH-CD3 trial. In this multicenter, randomized, double-blind, placebo-controlled trial, a single dose of RBL was compared with a single dose of placebo in 267 patients with first or subsequent CDI recurrence (CDI was diagnosed using any stool assay at the discretion of the local investigator).48 The primary end point was treatment success, defined as the absence of CDI diarrhea within 8 weeks of study treatment.48 At 8 weeks, using a Bayesian analysis leveraging the phase 2 data, RBL was superior to placebo, with success rates of 71% and 58%, respectively (posterior probability of superiority, 0.991; Figure 11).48
In analyses of gut microbes, RBL-treated responders showed increases in Bacteroidia and Clostridia species as well as decreases in Gammaproteobacteria and Bacilli classes.49 Metabolic analyses also indicated a restoration of the bile salt milieu that was less favorable for spore germination and CDI recurrence.50 Safety analyses revealed no concerning safety signals; the most common side effects were mild to moderate and short-lived (<10 days), including abdominal pain, diarrhea, distension, flatulence, and nausea.47
The PUNCH CD3 open-label study assessed the efficacy of RBL in a patient population that included individuals with common comorbidities, such as IBS and IBD.51 In an 8-week interim analysis of 300 patients, overall efficacy was 75%.51 Among responders, 84% had sustained treatment success at 24 weeks.51 Treatment with RBL was well tolerated, with no new safety signals identified.51
LBP Administration: RBL
RBL can be administered in any exam room. Of note, LBPs are administered to prevent recurrence in patients treated and cured of an infection. Because patients are not experiencing active infection during treatment administration, they are not considered vectors for CDI and do not require additional infection prevention precautions. Patients who remain symptomatic after antimicrobial treatment should undergo further clinical characterization to identify the cause of the symptoms before LBP administration, as CDI is unlikely the explanation.
The administration set for RBL includes a tube, a clamp, and a bag containing the RBL suspension.47 The patient is placed in a gown and positioned in the left lateral decubitus or knee-to-chest position. The lubricated tube is inserted toward the umbilicus, and the clip is opened with the effluent flowing into the patient via gravity over 2 minutes. The patient is monitored for up to 15 minutes before going home.
Fecal Microbiota Spores, Live-brpk
The second FDA-approved LBP and the only orally administered LBP is fecal microbiota spores, live-brpk (Vowst). Although previously known by other names, including SER-109, we will refer to this LBP as VOS for the purpose of this discussion. This product is a narrow-spectrum bacterial spore suspension derived from human stool. The human stool samples undergo an ethanol purification process before being refined, filtered, and encapsulated to remove most fungi, parasites, viruses, and non–spore-forming bacteria and isolate-only Firmicutes spores.52 After SOC antimicrobial treatment, a gentle bowel lavage, and a washout period, 4 capsules of VOS are taken orally per day for 3 days for a full dose of 3×107 colony-forming units (CFU).52
Data for VOS were collected in the pivotal phase 3 ECOSPOR-III trial. ECOSPOR-III enrolled 182 patients with second CDI recurrence and beyond (diagnosed with cell culture cytotoxic neutralization or enzyme immunoassay) to assess the superiority of VOS compared with placebo for reducing the risk for CDI recurrence.53 Patients were treated with 10 to 21 days of vancomycin or fidaxomicin, had a washout period, a gentle bowel lavage with magnesium citrate, and then were randomized to receive either placebo or VOS.53 Patients were evaluated for recurrence at week 8. Safety data were collected through week 24.53
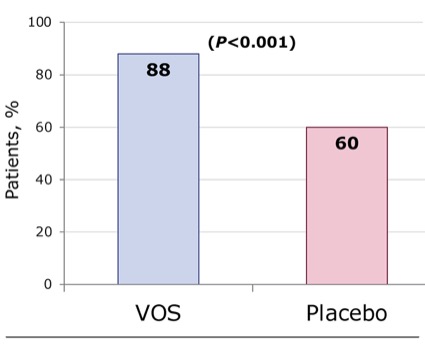
At 8 weeks, the primary efficacy end point was met: VOS was superior to placebo for reducing the risk for CDI recurrence. Twelve percent of the VOS-treated patients and 40% of placebo-treated patients experienced recurrence (Figure 12; relative risk, 0.32; 95% CI, 0.18-0.58; P<0.001).53 In addition, sustained clinical response at 8 weeks was 88% in the VOS-treated group and 60% in the placebo-treated group.53 In microbiome compositional assessments, compared with placebo, the VOS group showed an increase in the engraftment of Firmicutes species, including Ruminococcaceae and Lachnospiraceae, and decreases in the proinflammatory Enterobacteriaceae through week 8.53 In bile acid assessments, patients treated with VOS had a significantly higher level of secondary bile acids than those treated with placebo through week 8, consistent with a milieu that is less favorable for recurrence.53
No concerning safety signals were identified in safety analyses, with most side effects being mild to moderate and short-lived.53,54 The most common side effects were distension, fatigue, constipation, chills, and diarrhea.53 At the end of ECOSPOR-III, patients could roll over into an open-label extension (ECOSPOR-IV), which was expanded to enroll patients with first recurrence and beyond.53 Among 263 patients, recurrence rates were 8.7% at week 8 and 13.7% at week 24.53
LBP Administration: VOS
Before VOS administration, patients should complete SOC antimicrobial treatment and observe a washout period of 2 to 4 days.52 Patients should not eat or drink for 8 hours before the first dose of treatment and should consume 10 oz of magnesium citrate (or 250 mL polyethylene glycol–based bowel-cleansing product in those with renal insufficiency) for gentle bowel lavage ≥8 hours before taking the first dose.52 Four capsules of VOS are taken on an empty stomach daily for 3 days.52
Investigational LBP: VE303
VE303 is an orally administered agent that consists of an encapsulated formulation of 8 specific bacterial species (all commensal strains of Clostridia) originally derived from a healthy human intestinal microbiome.55 VE303 is synthetically produced using clonal cell banks and does not rely on human stool donation.55 VE303 was assessed in the phase 2 CONSORTIUM trial, in which patients were randomly assigned to treatment with high-dose VE303 (10 capsules daily for 14 days; total dose 1.1×1011 CFU), low-dose VE303 (2 capsules daily for 14 days), or placebo.55 Patients were followed for 8 weeks to evaluate recurrence and 24 weeks for safety assessments.55 Overall efficacy in the high-dose arm was 86% and 55% in the placebo arm (adjusted absolute risk reduction, 11%; 90% CI,
Case Study: Lorraine
Treatment
For her first recurrence, which occurred 2 months after her initial CDI, Lorraine was treated with oral vancomycin 125 mg daily for 10 days. For her second recurrence, which occurred 1 month later, Lorraine was treated with oral fidaxomicin 200 mg twice daily for 10 days.
Presentation: Lorraine responded initially to the treatment for her second recurrence, but 6 weeks later, her symptoms returned: 6 liquid (Bristol Scale, 7) bowel movements daily. Lorraine reported no recent travel, no recent sick contacts, no consumption of new foods, and no recent other medications or antimicrobials.
Commentary
Lorraine was unable to reconstitute her microbiome without assistance. Fidaxomicin shows the most benefit for treatment before the development of significant dysbiosis, so the panel believes there is not much value in giving fidaxomicin treatment at Lorraine’s stage of infection. Dr. Orenstein would treat Lorraine with vancomycin with LBP. Dr. Gonzales-Luna agreed with Dr. Orenstein’s decision to use LBP with antimicrobial treatment but would use fidaxomicin. It is important to remember that LBPs are adjunctive treatments and should always be administered with SOC antimicrobial therapy.
“Each and every clinician in this room should be comfortable and fully capable of prescribing either of these FDA-approved live biotherapeutic products. How do we present this to our patients? It’s actually relatively simple. We say, look, the reason that you’ve gotten recurrence or multiple recurrences of C. difficile is your microbiota isn’t quite healthy and it is having a hard time regrowing. So, all we’re going to do is give it what it needs to grow. We’re going to almost fertilize it or replace what is deficient there.”

Dr. Feuerstadt’s treatment algorithm for CDI in line with IDSA/SHEA guidelines is outlined on page 10 (Figure 13). For patients with their first episode of CDI, SOC vancomycin or fidaxomicin is used. If the patient has more than 2 risk factors for recurrence, add-on treatment with bezlotoxumab is considered. For patients with recurrence, strong consideration should be made for the EXTEND regimen of pulsed and tapered fidaxomicin or vancomycin taper. With first recurrence, in those with at least 2 risk factors for recurrence, consider adding bezlotoxumab during therapy or an LBP following therapy. For patients with a third episode (eg, second recurrence), treating with a standard antimicrobial course for 10 days followed by LBP is optimal.

- Craig M. CDC’s antibiotic resistance threats report, 2019. Accessed December 15, 2023. www.hhs.gov/sites/default/files/michael-craig-cdc-talk-Thursday-am-508.pdf
- Healthcare-associated infections—community interface report Clostridioides difficile infection. Centers for Disease Control and Prevention Emerging Infections Program. Accessed December 15, 2023. www.cdc.gov/hai/eip/Annual-CDI-Report-2020.html
- Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320-1330.
- Feuerstadt P, Theriault N, Tillotson G. The burden of CDI in the United States: a multifactorial challenge. BMC Infect Dis. 2023;23(1):132.
- Lurienne L, Bandinelli PA, Galvain T, Coursel CA, Oneto C, Feuerstadt P. Perception of quality of life in people experiencing or having experienced a Clostridioides difficile infection: a US population survey. J Patient Rep Outcomes. 2020;4(1):14.
- Khanna S, Pardi DS. Clostridium difficile infection: new insights into management. Mayo Clin Proc. 2012;87(11):1106-1117.
- Khanna S. Microbiota restoration for recurrent Clostridioides difficile: getting one step closer every day! J Intern Med. 2021;290(2):294-309.
- Davies K, Lawrence J, Berry C, et al. Risk factors for primary Clostridium difficile infection; results from the observational study of risk factors for Clostridium difficile infection in hospitalized patients with infective diarrhea (ORCHID). Front Public Health. 2020;8:293.
- Berenson CS, Lashner B, Korman LY, et al. Prevalence of comorbid factors in patients with recurrent Clostridioides difficile infection in ECOSPOR III, a randomized trial of an oral microbiota-based therapeutic. Clin Infect Dis. 2023;77(11):1504-1510.
- Song JH, Kim YS. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut Liver. 2019;13(1):16-24.
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787-8803.
- Ducarmon QR, Zwittink RD, Hornung BVH, et al. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol Mol Biol Rev. 2019;83(3):e00007-19.
- Bidell MR, Hobbs ALV, Lodise TP. Gut microbiome health and dysbiosis: a clinical primer. Pharmacotherapy. 2022;42(11):849-857.
- Buford TW. (Dis)trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5(1):80.
- Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13(9):508-516.
- Mullish BH, Allegretti JR. The contribution of bile acid metabolism to the pathogenesis of Clostridioides difficile infection. Therap Adv Gastroenterol. 2021;14:17562848211017725.
- Anthony WE, Wang B, Sukhum KV, et al. Acute and persistent effects of commonly used antibiotics on the gut microbiome and resistome in healthy adults. Cell Rep. 2022;39(2):110649.
- Bruno G, Zaccari P, Rocco G, et al. Proton pump inhibitors and dysbiosis: current knowledge and aspects to be clarified. World J Gastroenterol. 2019;25(22):2706-2719.
- Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740-748.
- Seto CT, Jeraldo P, Orenstein R, Chia N, DiBaise JK. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42.
- Zhang J, Zhang C, Zhang Q, et al. Meta-analysis of the effects of proton pump inhibitors on the human gut microbiota. BMC Microbiol. 2023;23(1):171.
- Isaac S, Scher JU, Djukovic A, et al. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother. 2017;72(1):128-136.
- Abujamel T, Cadnum JL, Jury LA, et al. Defining the vulnerable period for re-establishment of Clostridium difficile colonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One. 2013;8(10):e76269.
- Dawkins JJ, Allegretti JR, Gibson TE, et al. Gut metabolites predict Clostridioides difficile recurrence. Microbiome. 2022;10(1):87.
- Gonzales-Luna AJ, Skinner AM, Alonso CD, et al. Redefining Clostridioides difficile infection antibiotic response and clinical outcomes. Lancet Infect Dis. 2023;23(7):e259-e265.
- Johnson S, Gerding DN, Louie TJ, Ruiz NM, Gorbach SL. Sustained clinical response as an endpoint in treatment trials of Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother. 2012;56(8):4043-4045.
- Krutova M, Wilcox M, Kuijper E. Clostridioides difficile infection: are the three currently used antibiotic treatment options equal from pharmacological and microbiological points of view? Int J Infect Dis. 2022;124:118-123.
- Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology (Reading). 2010;156(pt 11):3216-3223.
- Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53(1):42-48.
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79(6):471-489.
- Yamaguchi T, Konishi H, Aoki K, Ishii Y, Chono K, Tateda K. The gut microbiome diversity of Clostridioides difficile-inoculated mice treated with vancomycin and fidaxomicin. J Infect Chemother. 2020;26(5):483-491.
- Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
- Guery B, Menichetti F, Anttila VJ, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (extend): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis. 2018;18(3):296-307.
- Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
- Mikamo H, Tateda K, Yanagihara K, et al. Efficacy and safety of fidaxomicin for the treatment of Clostridioides (Clostridium) difficile infection in a randomized, double-blind, comparative phase III study in Japan. J Infect Chemother. 2018;24(9):744-752.
- Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116(6):1124-1147.
- McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769-1775.
- Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305-317.
- Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis. 2018;67(5):649-656.
- Sehgal K, Zandvakili I, Tariq R, Pardi DS, Khanna S. Systematic review and meta-analysis: efficacy of vancomycin taper and pulse regimens in Clostridioides difficile infection. Expert Rev Anti Infect Ther. 2022;20(4):577-583.
- Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029-e1044.
- van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407-415.
- Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46(5):479-493.
- Kelly CR, Yen EF, Grinspan AM, et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT national registry. Gastroenterology. 2021;160(1):183-192.e183.
- Osman M, Budree S, Kelly CR, Panchal P, Allegretti JR, Kassam Z. Effectiveness and safety of fecal microbiota transplantation for Clostridioides difficile infection: results from a 5344-patient cohort study. Gastroenterology. 2022;163(1):319-322.
- Tariq R, Pardi DS, Khanna S. Resolution rates in clinical trials for microbiota restoration for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. Therap Adv Gastroenterol. 2023;16:17562848231174293.
- Rebyota (fecal microbiota, live-jslm) prescribing information. Ferring Pharmaceuticals; 2022.
- Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527-1538.
- Blount K, Walsh DM, Gonzalez C, Shannon B. 1064. Treatment success in reducing recurrent Clostridioides difficile infection with investigational live biotherapeutic RBX2660 is associated with microbiota restoration: consistent evidence from a phase 3 clinical trial. Open Forum Infect Dis. 2021;8(suppl 1):S624-S625.
- Papazyan R, Fuchs B, Blount K, Gonzalez C, Shannon B. 1039. Rapid restoration of bile acid compositions after treatment with RBX2660 for recurrent Clostridioides difficile infection—results from the PUNCH CD3 phase 3 trial. Open Forum Infect Dis. 2021;8(suppl 1):S610.
- Khanna S, Dubberke ER, Knapple WL, et al. An interim analysis of a phase 3, open-label study indicates efficacy and safety of RBX2660 in patients with recurrent Clostridioides difficile infection. Am J Gastroenterol. 2022:117(10S):e96-e96.
- Vowst (fecal microbiota spores, live-brpk) prescribing information. Braintree Laboratories, Inc.; 2023.
- Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. 2022;386(3):220-229.
- Korman L, Lashner B, Kraft C, et al. Fr572 24-week efficacy and safety data from ECOSPOR-III, a phase 3 double-blind, placebo-controlled randomized trial of SER-109, an investigational microbiome therapeutic for treatment of recurrent Clostridioides difficile infection. Gastroenterology. 2021;160(6):S-368-S-369.
- Louie T, Golan Y, Khanna S, et al. VE303, a defined bacterial consortium, for prevention of recurrent Clostridioides difficile infection: a randomized clinical trial. JAMA. 2023;329(16):1356-1366.
false
false


